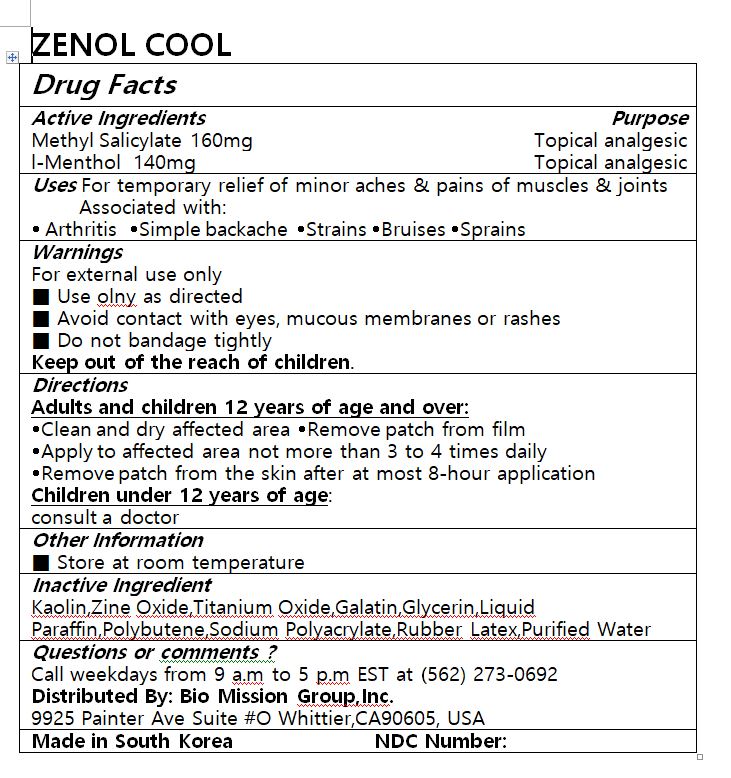
ZENOL COOL by Lydia Co., Ltd. Drug Facts
ZENOL COOL by
Drug Labeling and Warnings
ZENOL COOL by is a Otc medication manufactured, distributed, or labeled by Lydia Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZENOL COOL- methyl salicylate, l-menthol patch
Lydia Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Drug Facts
Kaolin,Zine Oxide,Titanium Oxide,Galatin,Glycerin,Liquid Paraffin,Polybutene,Sodium Polyacrylate,Rubber Latex,Purified Water
For temporary relief of minor aches & pains of muscles & joints Associated with:
Arthritis Simple backache Strains Bruises Sprains
Adults and children 12 years of age and over:
Clean and dry affected area Remove patch from film
Apply to affected area not more than 3 to 4 times daily
Remove patch from the skin after at most 8-hour application
Children under 12 years of age:
| ZENOL COOL
methyl salicylate, l-menthol patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Lydia Co., Ltd. (695735569) |
| Registrant - Lydia Co., Ltd. (695735569) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lydia Co., Ltd. | 695735569 | manufacture(72988-0026) , label(72988-0026) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.