SODIUM ACETATE injection, solution, concentrate
Sodium Acetate by
Drug Labeling and Warnings
Sodium Acetate by is a Prescription medication manufactured, distributed, or labeled by Hospira, Inc., Pfizer Healthcare India Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Sodium Acetate Injection, USP 40 mEq (2 mEq/mL) is a sterile, nonpyrogenic, concentrated solution of sodium acetate in water for injection. The solution is administered, after dilution, by the intravenous route as an electrolyte replenisher. It must not be administered undiluted. Each 20 mL contains 3.28 g of sodium acetate (anhydrous) which provides 40 mEq each of sodium (Na+) and acetate (CH3COO-). The solution contains no bacteriostat, antimicrobial agent or added buffer. May contain acetic acid for pH adjustment; the pH is 6.5 (6.0 to 7.0). The osmolar concentration is 4 mOsmol/mL (calc).
The solution is intended as an alternative to sodium chloride to provide sodium ion (Na+) for addition to large volume infusion fluids for intravenous use.
Sodium Acetate, USP (anhydrous) is chemically designated CH3COONa, a hygroscopic powder very soluble in water.
The semi-rigid container is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
-
CLINICAL PHARMACOLOGY
Sodium is the principal cation of extracellular fluid. It comprises more than 90% of total cations at its normal plasma concentration of approximately 140 mEq/liter. The sodium ion exerts a primary role in controlling total body water and its distribution.
Acetate (CH3COO-), a source of hydrogen ion acceptors, is an alternate source of bicarbonate (HCO3-) by metabolic conversion in the liver. This has been shown to proceed readily, even in the presence of severe liver disease.
-
INDICATIONS AND USAGE
Sodium Acetate Injection, USP 40 mEq is indicated as a source of sodium, for addition to large volume intravenous fluids to prevent or correct hyponatremia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions.
- CONTRAINDICATIONS
-
WARNINGS
Sodium Acetate Injection, USP 40 mEq must be diluted before use.
To avoid sodium overload and water retention, infuse sodium-containing solutions slowly.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
Solutions containing acetate ions should be used with great care in patients with metabolic or respiratory alkalosis. Acetate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
The intravenous administration of this solution (after appropriate dilution) can cause fluid and/or solute overloading resulting in dilution of other serum electrolyte concentrations, overhydration, congested states or pulmonary edema. Excessive administration of potassium free solutions may result in significant hypokalemia.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Sodium replacement therapy should be guided primarily by the serum sodium level.
Caution should be exercised in administering sodium-containing solutions to patients with severe renal function impairment, cirrhosis, cardiac failure, or other edematous or sodium-retaining states, as well as in patients with oliguria or anuria.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Solutions containing acetate ions should be used with caution as excess administration may result in metabolic alkalosis.
Pregnancy: Animal reproduction studies have not been conducted with sodium acetate. It is also not known whether sodium acetate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium acetate should be given to a pregnant woman only if clearly needed.
Pediatric Use: Safety and effectiveness have been established in the age groups infant to adolescent.
Geriatric Use: An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Sodium ions are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Sodium overload can occur with intravenous infusion of excessive amounts of sodium-containing solutions. See WARNINGS and PRECAUTIONS.
-
OVERDOSAGE
In the event of overdosage, discontinue infusion containing sodium acetate immediately and institute corrective therapy as indicated to reduce elevated serum sodium levels, and restore acid-base balance if necessary. See WARNINGS, PRECAUTIONS and ADVERSE REACTIONS.
-
DOSAGE AND ADMINISTRATION
Sodium Acetate Injection, USP 40 mEq is administered intravenously only after dilution in a larger volume of fluid. The dose and rate of administration are dependent upon the individual needs of the patient. Serum sodium should be monitored as a guide to dosage. Using aseptic technique, all or part of the contents of one or more vials may be added to other intravenous fluids to provide any desired number of milliequivalents (mEq) of sodium (Na+) with an equal number of acetate (CH3COO-).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. See PRECAUTIONS.
-
HOW SUPPLIED
Sodium Acetate Injection, USP 40 mEq (2 mEq/mL) is supplied as follows:
Unit of Sale Concentration Each NDC: 0409-7299-25
Tray Containing 25 Units40 mEq/20 mL
(2 mEq/mL)NDC: 0409-7299-45
Single-dose Fliptop VialEach vial is partially filled to provide air space for complete vacuum withdrawal of the contents into the intravenous container. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Manufactured by
Hospira, Inc., Lake Forest, IL 60045 USA
N+ and NOVAPLUS are registered trademarks of Vizient, Inc.LAB-1191-2.0
Revised: 01/2018
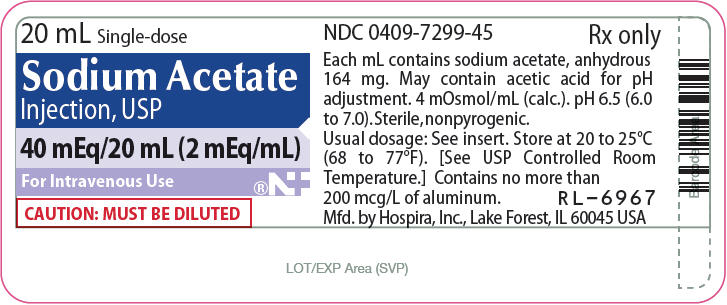
- PRINCIPAL DISPLAY PANEL - 20 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 20 mL Vial Tray
25 Units/NDC: 0409-7299-25
20 mL Single-doseSodium Acetate
Injection, USP40 mEq/20 mL (2 mEq/mL)
For Intravenous Use
Rx only
CAUTION:
MUST BE DILUTEDNOVAPLUS®

-
INGREDIENTS AND APPEARANCE
SODIUM ACETATE
sodium acetate injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0409-7299 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM ACETATE ANHYDROUS (UNII: NVG71ZZ7P0) (SODIUM CATION - UNII:LYR4M0NH37, ACETATE ION - UNII:569DQM74SC) SODIUM ACETATE ANHYDROUS 3.28 g in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ACETIC ACID (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-7299-25 25 in 1 TRAY 03/05/2014 1 NDC: 0409-7299-45 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018893 03/05/2014 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-7299) , LABEL(0409-7299) , MANUFACTURE(0409-7299) , PACK(0409-7299) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-7299) Establishment Name Address ID/FEI Business Operations Pfizer Healthcare India Private Limited 860037912 ANALYSIS(0409-7299) , LABEL(0409-7299) , MANUFACTURE(0409-7299) , PACK(0409-7299)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.