TOBAKIENT- ascorbic acid, cholecalciferol, riboflavin, pyridoxine hcl, folic acid, l-glutathione, astaxanthin capsule
Tobakient by
Drug Labeling and Warnings
Tobakient by is a Other medication manufactured, distributed, or labeled by Levins Pharmaceuticals, LLC, FORMULIFE, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PRINCIPAL DISPLAY PANEL
- STATEMENT OF IDENTITY
-
Health Claim
Dispensed by Prescription†
Tobakient™ is an orally administered prescription vitamin formulation for the clinical dietary management of suboptimal nutritional status in patients experiencing physiologically stressful conditions such as use of tobacco products, or to maintain levels when dietary intake of vitamins is inadequate or excretion or loss is excessive. ††
†This product is a prescription- folic acid with or without other dietary ingredients that – due to increased folic acid levels increased risk associated with masking of B12
deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760). The most appropriate way to ensure pedigree
reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription (Rx). This is not an Orange Book product.
This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The
ingredients, indication or claims of this product are not to be construed to be drug claims.1. Federal Register Notice of August 2, 1973 (38 FR 20750)
2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3. Federal Register Notice of March 5, 1996 (61 FR 8760)
†† These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
- WARNINGS
- PRECAUTIONS
-
DOSAGE & ADMINISTRATION
Usual adult dose is one (1) capsule once or twice daily or as prescribed by a licensed medical practitioner.
Tobakient™ capsules are supplied as (blue and white, oblong-shaped) capsules in a child-resistant bottle of 30-count ("71905-0100-30"**).
**Levins Pharmaceuticals does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
- SAFE HANDLING WARNING
-
INGREDIENTS AND APPEARANCE
TOBAKIENT
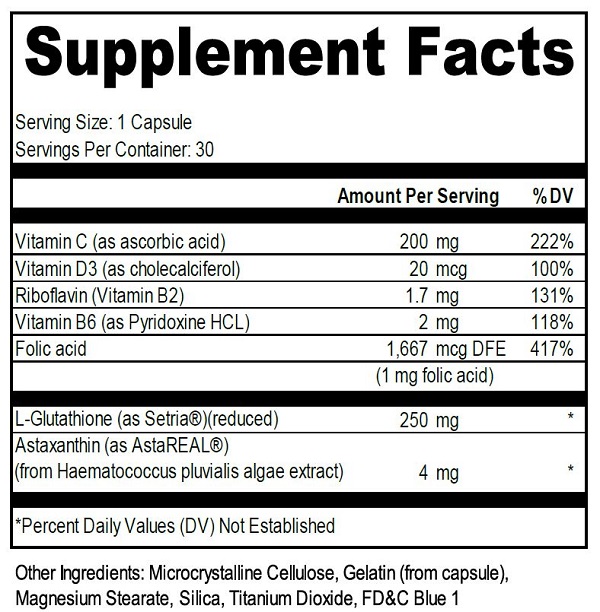
ascorbic acid, cholecalciferol, riboflavin, pyridoxine hcl, folic acid, l-glutathione, astaxanthin capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:71905-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.7 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 200 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 2 mg GLUTATHIONE (UNII: GAN16C9B8O) (GLUTATHIONE - UNII:GAN16C9B8O) GLUTATHIONE 250 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 20 ug ASTAXANTHIN (UNII: 8XPW32PR7I) (ASTAXANTHIN - UNII:8XPW32PR7I) ASTAXANTHIN 4 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1667 ug Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) GELATIN (UNII: 2G86QN327L) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71905-100-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 03/04/2019 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value size (solid drugs) 21 mm scoring 1 color color shape Labeler - Levins Pharmaceuticals, LLC (080839485) Registrant - Levins Pharmaceuticals, LLC (080839485) Establishment Name Address ID/FEI Business Operations FORMULIFE, INC. 024442806
Trademark Results [Tobakient]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TOBAKIENT 88197966 5830343 Live/Registered |
Levins Pharmaceuticals, LLC 2018-11-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.