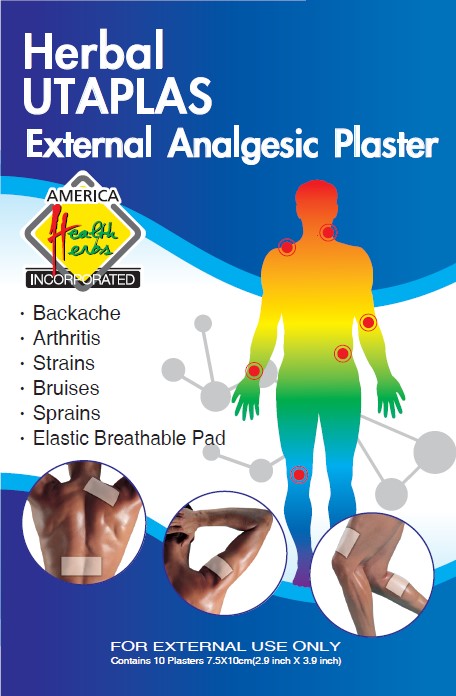
Herbal UTAPLAS External Analgesic Plaster
Herbal Utaplas External Analgesic Plaster by
Drug Labeling and Warnings
Herbal Utaplas External Analgesic Plaster by is a Otc medication manufactured, distributed, or labeled by Big 5 Nutrition LLC, TAIWAN SHUENN-AN BIOTECHNOLOGY PHARMACEUTICAL CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HERBAL UTAPLAS EXTERNAL ANALGESIC PLASTER- menthol, methyl salicylate patch
Big 5 Nutrition LLC
----------
Herbal UTAPLAS External Analgesic Plaster
Uses
For the temporary relief of minor aches and pains of muscles and joints due to:
● arthritis ● strains ● bruises ● sprains ● simple backache
Warnings
For external use only
Do not use
● on wounds ● irritated or damaged skin ● sensitive skin
● with a heating pad ● if pregnant
● with, or as the same time as, other external analgesic products
● if allergic to aspirin or salicylates
● if allergic to any ingredients of this product.
When using this product
● avoid contact with the eyes
● do not bandage tightly
● do not apply to wounds or damaged skin
● do not use more than 3 plasters a day
● do not use 1 hour before or after bathing
Directions
● Adults and children 12 years of age and older: clean and dry affected area.
Remove plaster from the protective layer, apply to the affected area, rub the area
with your hand to secure the patch.
● Plaster will remain effective for 8 hours.
● Avoid using the same patch on the same area for more than 2 days.
● Do not apply to area with excessive hair. Adhesive plaster may hurt skin upon
removal.
● Wet patch with warm water before removing from skin
● Children under 12 years of age: Do not use or consult a doctor.
Other Information
●Store unused patch in the pouch
●Store at room temperature, 20 to 25°C(68 to 77°F)
| HERBAL UTAPLAS EXTERNAL ANALGESIC PLASTER
menthol, methyl salicylate patch |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Big 5 Nutrition LLC (114574559) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TAIWAN SHUENN-AN BIOTECHNOLOGY PHARMACEUTICAL CO., LTD. | 656348265 | manufacture(82198-0004) , pack(82198-0004) , label(82198-0004) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.