VETAMEG- flunixin meglumine injection
VetaMeg by
Drug Labeling and Warnings
VetaMeg by is a Animal medication manufactured, distributed, or labeled by Aspen Veterinary Resources, Bimeda, Inc., Division of Cross Vetpharm Group, Bimeda-MTC Animal Health, Division of Cross Vetpharm Group. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Description
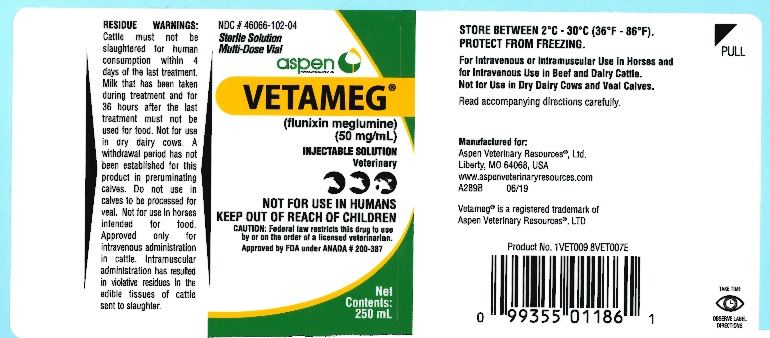
Approved by FDA under ANADA #200-387
VetamegTM
(flunixin meglumine)
Injectable Solution
50 mg/mL Sterile Solution
Veterinary Multi-Dose Vial
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
For Intravenous or Intramuscular Use in Horses and for Intravenous Use in Beef and Dairy Cattle. Not for Use in Dry Dairy Cows and Veal Calves.
CAUTION
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION
Each milliliter of VetamegTM Injectable Solution contains flunixin meglumine equivalent to 50 mg flunixin, 0.1 mg edetate disodium, 2.2 mg sodium formaldehyde sulfoxylate, 4.0 mg diethanolamine, 207.2 mg propylene glycol, 5.0 mg phenol as preservative, hydrochloric acid, water for injection q.s.
-
Pharmacology
PHARMACOLOGY
Flunixin meglumine is a potent, non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is significantly more potent than pentazocine, meperidine, and codeine as an analgesic in the rat yeast paw test.
Horse: Flunixin is four times as potent on a mg-per-mg basis as phenylbutazone as measured by the reduction in lameness and swelling in the horse. Plasma half-life in horse serum is 1.6 hours following a single dose of 1.1 mg/kg. Measurable amounts are detectable in horse plasma at 8 hours postinjection.
Cattle: Flunixin meglumine is a weak acid (pKa=5.82)1 which exhibits a high degree of plasma protein binding (approximately 99 percent).2 However, free (unbound) drug appears to readily partition into body tissues (Vss prediction range from 297 to 782 mL/kg.2-5 Total body water is approximately equal to 570 mL/kg).6 In cattle, elimination occurs primarily through biliary excretion.7 This may, at least in part, explain the presence of multiple peaks in the blood concentration/time profile following IV administration.2 In healthy cattle, total body clearance has been reported to range from 90 to 151 mL/kg/hr.2-5 These studies also report a large discrepancy between the volume of distribution at steady state (Vss) and the volume of distribution associated with the terminal elimination phase (Vss) and the volume of distribution associated with the terminal elimination phase (Vß). This discrepancy appears to be attributable to extended drug elimination from a deep compartment.8 The terminal half-life has been shown to vary from 3.14 to 8.12 hours.2-5 Flunixin persists in inflammatory tissues9 and is associated with anti-inflammatory properties which extend well beyond the period associated with detectable plasma drug concentrations.4,9 These observations account for the counterclockwise hysteresis associated with flunixin's pharmacokinetic/pharmacodynmic relationships.10
Therefore, prediction of drug concentrations based upon the estimated plasma terminal elimination half-life will likely underestimate both the duration of drug action and the concentration of drug remaining at the site of activity.
-
Indications
INDICATIONS
Horse: VetamegTM Injectable Solution is recommended for the alleviation of inflammation and pain associated with musculoskeletal disorders in the horse. It is also recommended for the alleviation of visceral pain associated with colic in the horse.
Cattle: VetamegTM Injectable Solution is indicated for the control of pyrexia associated with bovine respiratory disease, endotoxemia and acute bovine mastitis. VetamegTM Injectable Solution is also indicated for the control of inflammation in endotoxemia.
-
Dose and Administration
DOSE AND ADMINISTRATION
Horse: The recommended dose for musculoskeletal disorders is 0.5 mg per pound (1 mL/100 lbs) of body weight once daily. Treatment may be given by intravenous or intramuscular injection and repeated for up to 5 days. Studies show onset of activity is within 2 hours. Peak response occurs between 12 and 16 hours and duration of activity is 24-36 hours.
The recommended dose for the alleviation of pain associated with equine colic is 0.5 mg per pound of body weight. Iintravenous administration is recommended for prompt relief. Clincial studies show pain is alleviated in less than 15 minutes in many cases. Treatment may be repeated when signs of colic recur. During clincial studies approximately 10% of the horses required one or two additional treatments. The cause of colic should be determined and treated with concomitant therapy.
Cattle: The recommended dose for control of pyrexia associated with bovine respiratory disease and endotoxemia and control of inflammation in endotoxemia is 1.1 to 2.2 mg/kg (0.5 to 1.0 mg/lb; 1 to 2 mL per 100 lbs) of body weight given by slow intravenous administration either once a day as a single dose or divided into two doses administered at 12-hour intervals for up to 3 days. The total daily dose should not exceed 2.2 mg/kg (1.0 mg/lb) of body weight. Avoid rapid intravenous administration of the drug. The recommended dose for acute bovine mastitis is 2.2 mg/kg (1 mg/lb; 2 mL per 100 lbs) of body weight given once by intravenous administration.
-
Contraindications
CONTRAINDICATIONS
Horse: There are no known contraindications to this drug when used as directed. Intra-arterial injection should be avoided. horses inadvertently injected intra-arterially can show adverse reactions. Signs can be ataxia, incoordination, hyperventilation, hysteria, and muscle weakness. Signs are transient and disappear without antidotal medication within a few minutes. Do not use in horses showing hypersensitivity to flunixin meglumine.
Cattle: NSAIDs inhibit production of prostaglandins which are important in signaling the initiation of parturition. The use of flunixin can delay parturition and prolong labor which may increase the risk of still birth. Do not use VetamegTM Injectable Solution within 48 hours of expected parturition. Do not use in animals showing hypersensitivity to flunixin meglumine. Use judiciously when renal impairment or gastric ulceration are suspected.
-
Residue Warning
RESIDUE WARNINGS: Cattle must not be slaughtered for human consumption within 4 days of the last treatment. Milk that has been taken during treatment and for 36 hours after the last treatment must not be used for food. Not for use in dry dairy cows. A withdrawal period has not been established for this product in preruminating calves. Do not use in calves to be processed for veal. Not for use in horses intended for food. Approved only for intravenous administration in cattle. Intramuscular administration has resulted in violative residues in the edible tissues of cattle sent to slaughter.
-
Precautions
PRECAUTIONS
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal and renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual patient. Patients at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfuction. Since many NSAIDs possess the potential to induce gastrointestinal ulceration, concomitant use of VetamegTM Injectable Solution with other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided or closely monitored.
Horse: The effect of VetamegTM Injectable Solution on pregnancy has not been determined. Studies to determine activity of VetamegTM Injectable Solution when administered concomitantly with other drugs have not been conducted. Drug compatibility should be monitored closely in patients requiring adjunctive therapy.
Cattle: Do not use in bulls intended for breeding, as reproductive effects of VetamegTM Injectable Solution in these classes of cattle have not been investigated. NSAIDs parturition and the estrous cycle. There may be delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. The effects of flunixin on imminent parturition have not been evaluated in a controlled study. NSAIDs are known to have the potential to delay parturition through a tocolytic effect. Do not exceed the recommended dose.
-
SAFETY
SAFETY
Horse: A 3-fold intramuscular dose of 1.5 mg/lb of body weight daily for 10 consecutive days was safe. No changes were observed in hematology, serum chemistry, or urinalysis values. Intravenous dosages of 0.5 mg/lb daily for 15 days; 1.5 mg/lb daily for 10 days; and 2.5 mg/lb daily for 5 days produced no changes in blood or urine parameters. No injection site irritation was observed following intramuscular injection of the 0.5 mg/lb recommended dose. Some irritation was observed following a 3-fold dose administered intramuscularly.
Cattle: No flunixin-related changes (adverse reactions) were noted in cattle administered a 1X (2.2 mg/kg; 1.0 mg/lb) dose for 9 days three times the maximum clinical duration. Minimal toxicity manifested itself at moderately elevated doses (3X and 5X) when flunixin was administered daily for 9 days, with occasional findings of blood in the feces and/or urine. Discontinue use if hematuria or fecal blood are observed.
-
Adverse Reactions
ADVERSE REACTIONS
In horses, isolated reports of local reactions following intramuscular injection, particularly in the neck, have been received. These include localized swelling, sweating, induration, and stiffness. In rare instances in horses, fatal or nonfatal clostridial infections or other infections have been reported in association with intramuscular use of flunixin meglumine. In horses and cattle, rare instances of anaphylactic-like reactions, some of which have been fatal, have been reported, primarily following intravenous use.
- How Supplied
- Storage
-
References
REFERENCES
1. Johansson M, Anler EL. Gas chromatographic analysis of flunixin in equine urine after extractive methylation. J Chromatogr. 1988;427:55-66
2. Odensvik K, Johansson M. High-performance liquid chromatography method for determination of flunixin in bovine plasma and pharmacokinetics after single and repeated doses of the drug. Am J Vet Res. 1995;56:489-495.
3. Anderson KL, Neff-Davis CA, Davis LE Bass VD. Pharmacokinetics of flunixin meglumine in lactating cattle after single and multiple intramuscular and intravenous administrations. Am J Vet Res. 1990;51:1464-1467.
4. Odensvik K. Pharmacokinetics of flunixin and its effect on prostaglandin F2a metabolite concentrations after oral and intravenous administration in heifers. J Vet Pharmacol Ther. 1995;18:254-259.
5. Hardee GE, Smith JA, Harris SJ. Pharmacokinetics of flunixin meglumine in the cow. Res Vet Sci. 1985;39:110-112.
6. Ruckebusch Y, Pheneuf LP, Dunlop R. Physiology of Small and Large Animals. Chapter 2: "Body Fluid Compartments" Philadelphia, Pa: B.C. Decker; 1991:8-18.
7. Kopcha M, Ahl AS. Experimental uses of flunixin meglumine and phenylbutazone in food-producing animals. J Am Vet Med Assoc. 1989;194:45-49.
8. Wagner JG. Significance of ratios of different volumes of distribution in pharmacokinetics. Biopharm & Drug Dispos. 1983;4:263-270.
9. Lees P, Higgins AJ. Flunixin inhibits prostaglandin E2 production in equine inflammation. Res Vet Sci. 1984;37:347-349.
10. Landoni MF, Cunningham FM, Lees P. Determination of pharmacokinetics and pharmacodynamics of flunixin in calves by use of pharmacokinetic/pharmacodynamic modeling. Am J Vet Res. 1995;56:786-794.
- Package Label Principle Display Panel
-
INGREDIENTS AND APPEARANCE
VETAMEG
flunixin meglumine injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 46066-102 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength flunixin meglumine (UNII: 8Y3JK0JW3U) (FLUNIXIN - UNII:356IB1O400) FLUNIXIN 50 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46066-102-03 100 mL in 1 VIAL, MULTI-DOSE 2 NDC: 46066-102-04 250 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200387 03/02/2006 Labeler - Aspen Veterinary Resources (627265361) Registrant - Bimeda, Inc., Division of Cross Vetpharm Group (060492923) Establishment Name Address ID/FEI Business Operations Bimeda-MTC Animal Health, Division of Cross Vetpharm Group 256232216 manufacture Establishment Name Address ID/FEI Business Operations Zhejiang Hisoar Pharmaceutical Co., Ltd. 530736917 api manufacture Establishment Name Address ID/FEI Business Operations Excella, GmBH 329809800 api manufacture
Trademark Results [VetaMeg]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VETAMEG 88245277 not registered Live/Pending |
Aspen Veterinary Resources, Ltd. 2018-12-29 |
 VETAMEG 77134380 3375169 Dead/Cancelled |
Aspen Veterinary Resources, Ltd. 2007-03-19 |
 VETAMEG 76486825 not registered Dead/Abandoned |
Aspen Veterinary Resources, Ltd. 2003-02-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.