LEADER LIDOCAINE- lidocaine hcl liquid
Leader Lidocaine by
Drug Labeling and Warnings
Leader Lidocaine by is a Otc medication manufactured, distributed, or labeled by CARDINAL HEALTH, Weeks & Leo Co., Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- Directions
- Other information
-
INACTIVE INGREDIENT
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis (aloe vera) leaf juice, aminomethyl propanol,
C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, edetate disodium, ethylhexylglycerin, glyceryl monostearate, methylparaben, purified water, SD alcohol 40, steareth-21 - QUESTIONS
-
PRINCIPAL DISPLAY PANEL
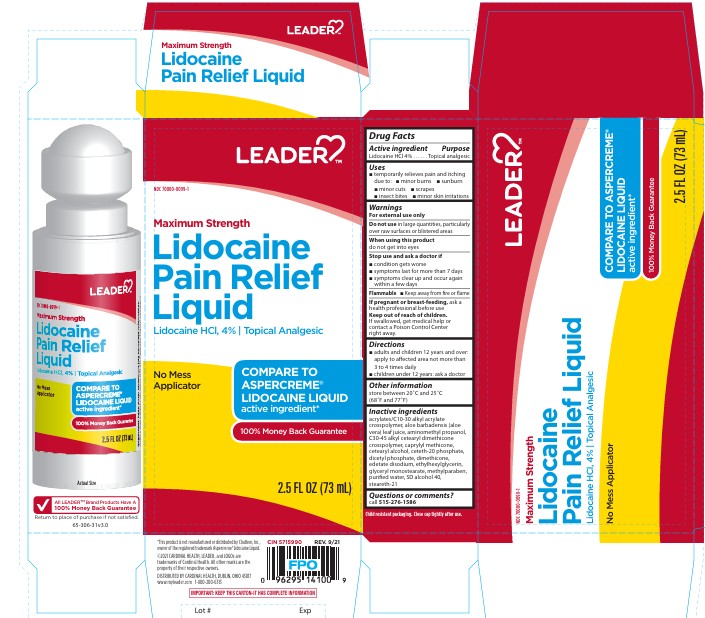 Leader
Leader
NDC: 70000-0099-1
Maximum Strength
Lidocaine
Pain Relief
Liquid
Lidocaine HCl, 4% Topical Analgesic
No Mess
Applicator
Compare to
Aspercreme
Lidocaine liquid
active ingredient
100% Money Back Guarantee
2.5 Fl oz (73 mL)
-
INGREDIENTS AND APPEARANCE
LEADER LIDOCAINE
lidocaine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70000-0099 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CETETH-20 PHOSPHATE (UNII: 921FTA1500) STEARETH-21 (UNII: 53J3F32P58) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) METHYLPARABEN (UNII: A2I8C7HI9T) DICETYL PHOSPHATE (UNII: 2V6E5WN99N) DIMETHICONE 350 (UNII: 2Y53S6ATLU) ALCOHOL (UNII: 3K9958V90M) ALOE VERA LEAF (UNII: ZY81Z83H0X) CETEARYL ALCOHOL (UNII: 2DMT128M1S) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) CAPRYLYL METHICONE (UNII: Q95M2P1KJL) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) C30-45 ALKYL CETEARYL DIMETHICONE CROSSPOLYMER (UNII: 4ZK9VP326R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70000-0099-1 1 in 1 CARTON 10/20/2021 1 73 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/20/2021 Labeler - CARDINAL HEALTH (063997360) Registrant - Weeks & Leo Co., Inc. (005290028) Establishment Name Address ID/FEI Business Operations Weeks & Leo Co., Inc. 005290028 manufacture(70000-0099)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.