ReBoost Cherry Throat Spray
ReBoost by
Drug Labeling and Warnings
ReBoost by is a Homeopathic medication manufactured, distributed, or labeled by MediNatura. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REBOOST- apis mellifera, asclepias tuberosa root, atropa belladonna, spray
MediNatura
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
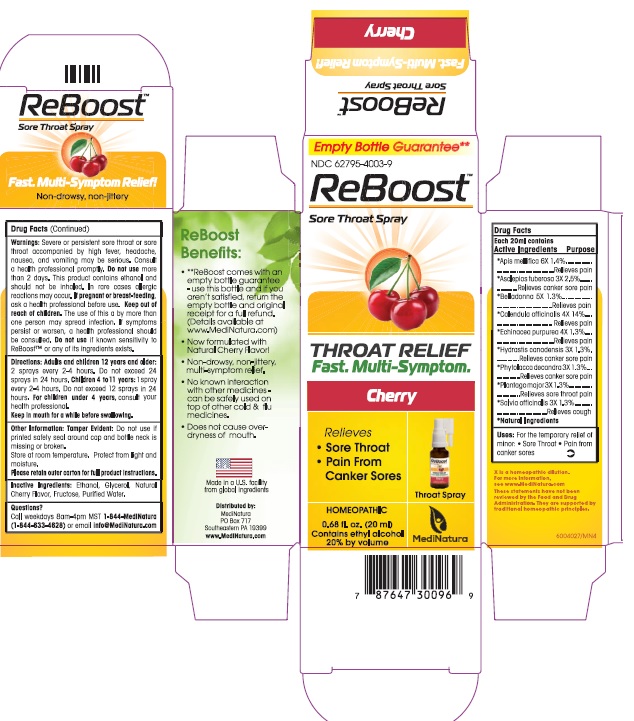
ReBoost Cherry Throat Spray
ACTIVE INGREDIENTS
Each 20ml contains
Active Ingredients Purpose
*Apis mellifica 6X 1.4%........ ...................Relieves pain
*Asclepias tuberosa 3X 2.5%.................. Relieves pain from canker sores
*Belladonna 5X 1.3%...............................Relieves pain
*Calendula officinalis 4X 14%...................Relieves pain
*Echinacea purpurea 4X 1.3%...................Relieves pain
*Hydrastis canadensis 3X 1.3%.................Relieves pain from canker sores
*Phytolacca decandra 3X 1.3%.................Relieves pain from canker sores
*Plantago major 3X 1.3%.........................Relieves sore throat pain
*Salvia officinalis 3X 1.3%.........................Relieves tickling cough
*Natural Ingredients
DIRECTIONS
Directions: Adults and children 12 years and older: 2 sprays every 2-4 hours. Do not exceed 24 sprays in in 24 hours.
Children 4 to 11 years: 1 spray every 2-4 hours. Do not exceed 12 sprays into in 24 hours
For children under 4 years, consult your health professional.
Keep in mouth for a while before swallowing
WARNINGS
Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a health professional promptly. Do not use more than 2 days. This product contains ethanol and should not be inhaled. In rare cases allergicreactions may occur. If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. The use of this a by more than one person may spread infection. If symptoms persist or worsen, a health professional should be consulted. Do not use if known sensitivity to ReBoost™ or any of its ingredients exists.
| REBOOST
apis mellifera, asclepias tuberosa root, atropa belladonna, spray |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - MediNatura (079324099) |