BAYTRIL OTIC- enrofloxacin/silver sulfadiazine emulsion
Baytril Otic by
Drug Labeling and Warnings
Baytril Otic by is a Animal medication manufactured, distributed, or labeled by Bayer HealthCare LLC Animal Health Division. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
PRODUCT DESCRIPTION:
Each milliliter of Baytril® Otic contains: enrofloxacin 5 mg (0.5% w/v), silver sulfadiazine (SSD) 10 mg (1.0% w/v), benzyl alcohol (as a preservative) and cetylstearyl alcohol (as a stabilizer) in a neutral oil and purified water emulsion. The active ingredients are delivered via a physiological carrier (a nonirritating emulsion).
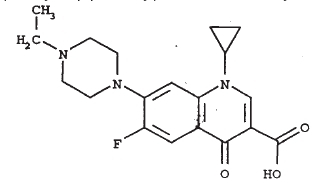
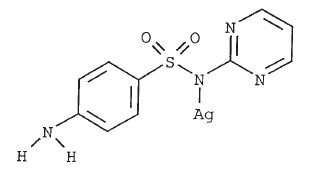
- CHEMICAL NOMENCLATURE AND STRUCTURE:
-
ACTIONS:
Enrofloxacin, a 4-fluoroquinolone compound, is bactericidal with activity against a broad spectrum of both Gram negative and Gram positive bacteria. Fluoroquinolones elicit their bactericidal activities through interactions with two intracellular enzymes, DNA gyrase (DNA topoisomerase II) and DNA topoisomerase IV, which are essential for bacterial DNA transcription, synthesis and replication. It is believed that fluoroquinolones actively bind with bacterial DNA:ENZYME complexes and thereby inhibit the essential processes catalyzed by the enzymes (DNA supercoiling and chromosomal decatenation).1 The ultimate outcome of the fluoroquinolone intervention is DNA fragmentation and bacterial cell death.2,3
Silver sulfadiazine (SSD) is synthesized from silver nitrate and sodium sulfadiazine.4 This compound has a wide spectrum of antimicrobial activity against Gram negative and Gram positive bacteria and is also an effective antimycotic.5,6 SSD suppresses microbial growth through inhibition of DNA replication and modification of the cell membrane.
-
MICROBIOLOGY:
In clinical field trials, Baytril® Otic demonstrated elimination or reduction of clinical signs associated with otitis externa and in vitro activity against cultured organisms. Baytril® Otic is effective when used as a treatment for canine otitis externa associated with one or more of the following organisms: Malassezia pachydermatis, coagulase-positive Staphylococcus spp.,Pseudomonas aeruginosa, Enterobacter spp., Proteus mirabilis, Streptococci spp., Aeromonas hydrophila, Aspergillus spp., Klebsiella pneumoniae, and Candida albicans.
In vitro assays, such as disk-diffusion and agar/broth-dilution, are used to determine the susceptibilities of microbes to antimicrobial therapies. Results of agar/broth-dilution assays are reported as a Minimal Inhibitory Concentration (MIC) which represents the lowest antimicrobial concentration, expressed in μg/mL, capable of inhibiting the growth of a pathogenic microorganism. MICs are used in conjunction with pharmacokinetics to predict the in vivo efficacy of systemically administered antimicrobials. Topical administration of Baytril® Otic to an exudate and debris-free canal, however, will generally result in local antimicrobial concentrations that greatly exceed serum and tissue levels resulting from systemic therapy. Therefore, when using Baytril® Otic as a treatment for canine otitis externa, interpret susceptibility data cautiously.
-
INDICATIONS:
Baytril® Otic is indicated as a treatment for canine otitis externa complicated by bacterial and fungal organisms susceptible to enrofloxacin and/or silver sulfadiazine (see Microbiology section).
-
EFFECTIVENESS:
Due to its combination of active ingredients, Baytril® Otic provides antimicrobial therapy against bacteria and fungi (which includes yeast) commonly encountered in cases of canine otitis externa.
The effectiveness of Baytril® Otic was evaluated in a controlled, double-blind, multi-site clinical trial. One hundred and sixty-nine dogs (n=169), with naturally occurring active otitis externa participated in the study. The presence of active disease was verified by aural cytology, microbial culture and otoscopy/clinical scoring. Qualified cases were randomly assigned to either Baytril Otic treatment (n=113) or to a comparable placebo-based regimen (n=56).
Treatments were administered twice daily for up to 14 days. Assessment of effectiveness was based on continued resolution of clinical signs 3 to 4 days following administration of the last dose.
At study conclusion, Baytril® Otic was found to be a significantly more effective treatment for canine otitis externa than the placebo regimen. Based on the scoring system used to assess treatment response, therapeutic success occurred in 67% of Baytril® Otic-treated infections compared to 14% with placebo (r-value2 0.001) after 14 days of treatment.
- CONTRAINDICATIONS:
-
HUMAN WARNINGS:
Not for human use. Keep out of the reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation develops or persists following ocular or dermal exposures. Individuals with a history of hypersensitivity to quinolone compounds or antibacterials should avoid handling this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight.
-
PRECAUTIONS:
The use of Baytril® Otic in dogs with perforated tympanic membranes has not been evaluated. Therefore, the integrity of the tympanic membrane should be evaluated before administering this product. If hearing or vestibular dysfunction is noted during the course of treatment, discontinue use of Baytril® Otic.
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures.
Quinolone-class drugs have been associated with cartilage erosions in weightbearing joints and other forms of arthropathy in immature animals of various species.
The safe use of Baytril® Otic in dogs used for breeding purposes, during pregnancy, or in lactating bitches, has not been evaluated.
-
ADVERSE REACTIONS:
During clinical trials, 2 of 113 (1.7%) dogs exhibited reactions that may have resulted from treatment with Baytril® Otic.
Both cases displayed local hypersensitivity responses of the aural epithelium to some component within the Baytril® Otic formulation. The reactions were characterized by acute inflammation of the ear canal and pinna.
For medical emergencies or to report adverse reactions, call 1-800-422-9874. For customer service or to obtain product information, including Safety Data Sheet, call 1-800-633-3796.
-
SAFETY:
General Safety Study:
In a target animal safety study, Baytril® Otic was administered in both ears of 24 clinically normal beagle dogs at either recommended or exaggerated dosages: 10, 30 or 50 drops applied twice daily for 42 consecutive days. A control group of 8 beagle dogs was treated by administering 50 drops of vehicle in one ear twice daily for 42 consecutive days, with the contralateral ear untreated. Erythema was noted in all groups, including both treated and untreated ears in the controls, which resolved following termination of treatment.
Oral Safety Study:
In order to test safety in case of ingestion, Baytril® Otic was administered, twice daily for 14 consecutive days, to the dorsum of the tongue and to the left buccal mucosa of 6 clinically normal dogs. No adverse local or systemic reactions were reported.
-
DOSAGE AND ADMINISTRATION:
Shake well before each use.
Tilt head so that the affected ear is presented in an upward orientation. Administer a sufficient quantity of Baytril® Otic to coat the aural lesions and the external auditory canal. As a general guide, administer 5-10 drops per treatment in dogs weighing 35 lbs. or less and 10-15 drops per treatment in dogs weighing more than 35 lbs. Following treatment, gently massage the ear so as to ensure complete and uniform distribution of the medication throughout the external ear canal. Apply twice daily for a duration of up to 14 days.
- STORAGE:
- HOW SUPPLIED:
-
REFERENCES:
- 1. Hooper DC and Wolfson JS. Mechanisms of quinolone action and bacterial killing in quinolone antimicrobial agents. Washington DC, American Society for Microbiology, 2nd ed., 1993: 53-75.
- 2. Gootz TD and Brightly KE. Fluoroquinolone antibacterial: mechanism of action, resistance and clinical aspects. Medicinal Research Reviews 1996: 16 (5): 433-486.
- 3. Drlica K and Zhoa X. DNA gyrase, topoisomerase IV and the 4-quinolones. Microbiology and Molecular Biology Reviews 1997: 61(3): 377-392.
- 4. Fox CL. Silver sulfadiazine: a new topical therapy for Pseudomonas in burns. Archives of Surgery 1968: 96:184- 188.
- 5. Wlodkowski TJ and Rosenkranz HS. Antifungal activity of silver sulfadiazine. Lancet 1973: 2:739-740.
- 6. Schmidt A. In vitro activity of climbazole, clotrimazole and silver sulfadiazine against isolates of Malassezia pachydermatis. J of Vet Medicine Series B 1997: 44: 193-197.
- SPL UNCLASSIFIED SECTION
-
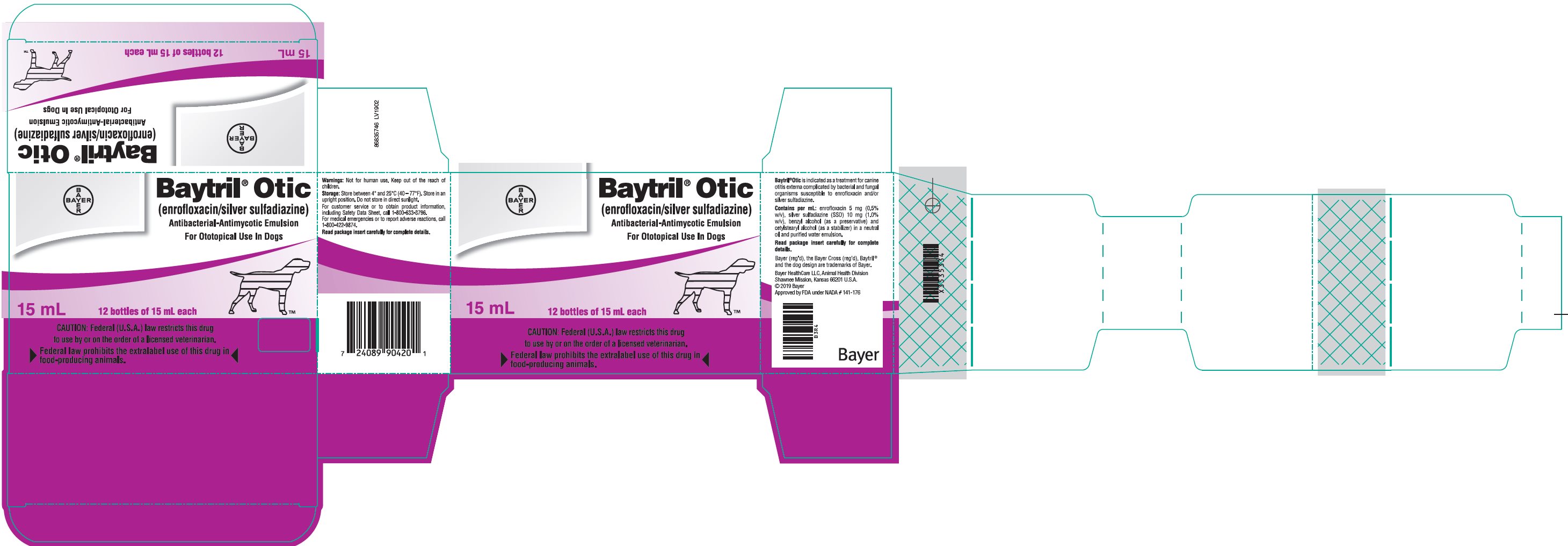
Principal Display Panel - carton label
Baytril® Otic
(enrofloxacin/silver sulfadiazine)
Antibacterial-Antimycotic Emulsion
For Ototopical Use in Dogs
15 mL
12 bottles of 15 mL each
CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.
►Federal law prohibits the extralabel use of this drug in food-producing animals.◄
-
Principal Display Panel - unit label
Baytril® Otic
(enrofloxacin/silver sulfadiazine)
Antibacterial-Antimycotic Emulsion
For Ototopical Use in Dogs
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
►Federal law prohibits the extralabel use of this drug in food-producing animals.◄
15 mL
Bayer HealthCare LLC, Animal Health Division
Shawnee Mission, Kansas 66201 U.S.A.
Approved by FDA under NADA # 141-17686835711 LV1902
B3R5
-
INGREDIENTS AND APPEARANCE
BAYTRIL OTIC
enrofloxacin/silver sulfadiazine emulsionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2286 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENROFLOXACIN (UNII: 3DX3XEK1BN) (ENROFLOXACIN - UNII:3DX3XEK1BN) ENROFLOXACIN 5 mg in 1 mL SILVER SULFADIAZINE (UNII: W46JY43EJR) (SULFADIAZINE - UNII:0N7609K889) SILVER SULFADIAZINE 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2286-03 12 in 1 CARTON 1 NDC: 0859-2286-01 15 mL in 1 BOTTLE, DROPPER 2 NDC: 0859-2286-05 6 in 1 CARTON 2 NDC: 0859-2286-01 15 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141176 09/29/2000 Labeler - Bayer HealthCare LLC Animal Health Division (152266193)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.