KIDS-EEZE- diphenhydramine hydrochloride tablet, orally disintegrating
Kids-EEZE by
Drug Labeling and Warnings
Kids-EEZE by is a Otc medication manufactured, distributed, or labeled by ProPhase Labs, Inc., Pharmaloz Manufacturing, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each soft chew)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- QUESTIONS
-
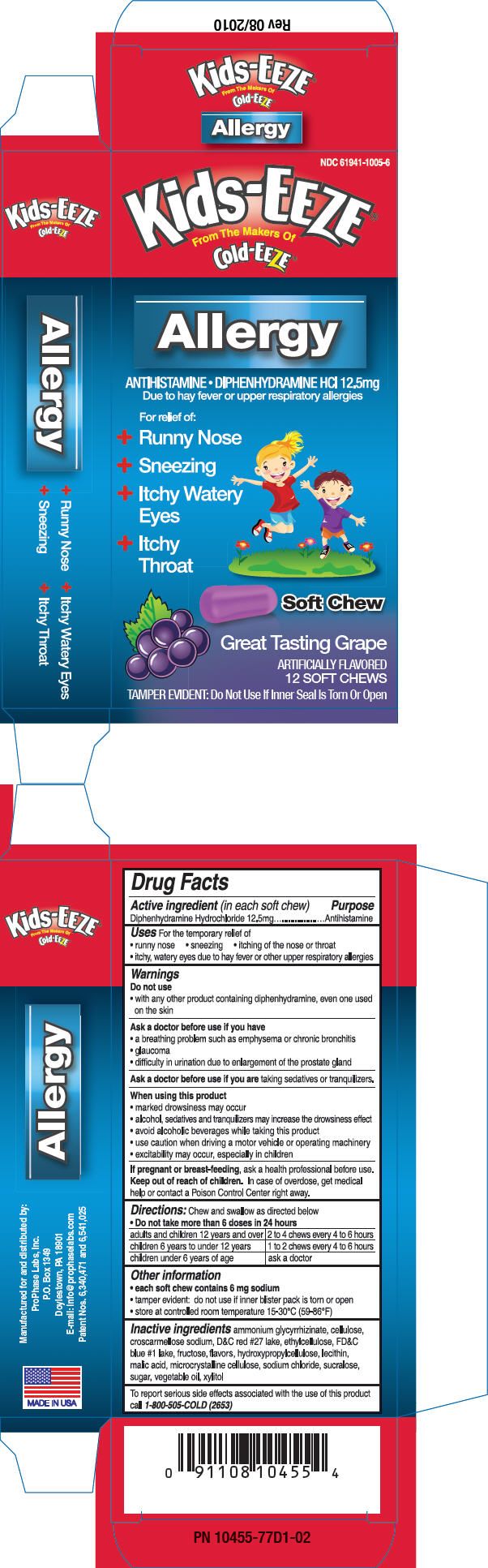
PRINCIPAL DISPLAY PANEL - 12.5 mg Package
NDC: 61941-1005-6
Kids-EEZE®
From The Makers Of
Cold-EEZE®Allergy
ANTIHISTAMINE DIPHENHYDRAMINE HCl 12.5mg
Due to hay fever or upper respiratory allergiesFor relief of:
- +
Runny Nose
- + Sneezing
- + Itchy Watery
Eyes- + Itchy
Throat - + Sneezing
Soft Chew
Great Tasting Grape
ARTIFICIALLY FLAVORED
12 SOFT CHEWSTAMPER EVIDENT: Do Not Use If Inner Seal Is Torn Or Open
- +
Runny Nose
-
INGREDIENTS AND APPEARANCE
KIDS-EEZE ALLERGY
diphenhydramine hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61941-1005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diphenhydramine Hydrochloride (UNII: TC2D6JAD40) (Diphenhydramine - UNII:8GTS82S83M) Diphenhydramine Hydrochloride 12.5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ETHYLCELLULOSE (4 MPA.S) (UNII: KC5472WRJK) FRUCTOSE (UNII: 6YSS42VSEV) MALIC ACID (UNII: 817L1N4CKP) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) Product Characteristics Color PURPLE Score no score Shape OVAL Size 17mm Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61941-1005-1 72 in 1 CASE 1 NDC: 61941-1005-6 12 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 09/01/2010 Labeler - ProPhase Labs, Inc. (620557298) Establishment Name Address ID/FEI Business Operations ProPhase Labs, Inc. 620557298 LABEL, ANALYSIS Establishment Name Address ID/FEI Business Operations Pharmaloz Manufacturing, Inc. 067101998 MANUFACTURE, ANALYSIS, PACK, REPACK
Trademark Results [Kids-EEZE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KIDS-EEZE 86063000 4574810 Live/Registered |
ProPhase Labs, Inc. 2013-09-12 |
 KIDS-EEZE 78842320 3201772 Dead/Cancelled |
Quigley Corporation, The 2006-03-21 |
 KIDS-EEZE 75271291 2265413 Dead/Cancelled |
Quigley Corporation 1997-04-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.