HEPARIN SODIUM AND SODIUM CHLORIDE- heparin sodium injection, solution
Heparin Sodium and Sodium Chloride by
Drug Labeling and Warnings
Heparin Sodium and Sodium Chloride by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter Healthcare Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- HEALTH CARE PROVIDER LETTER
-
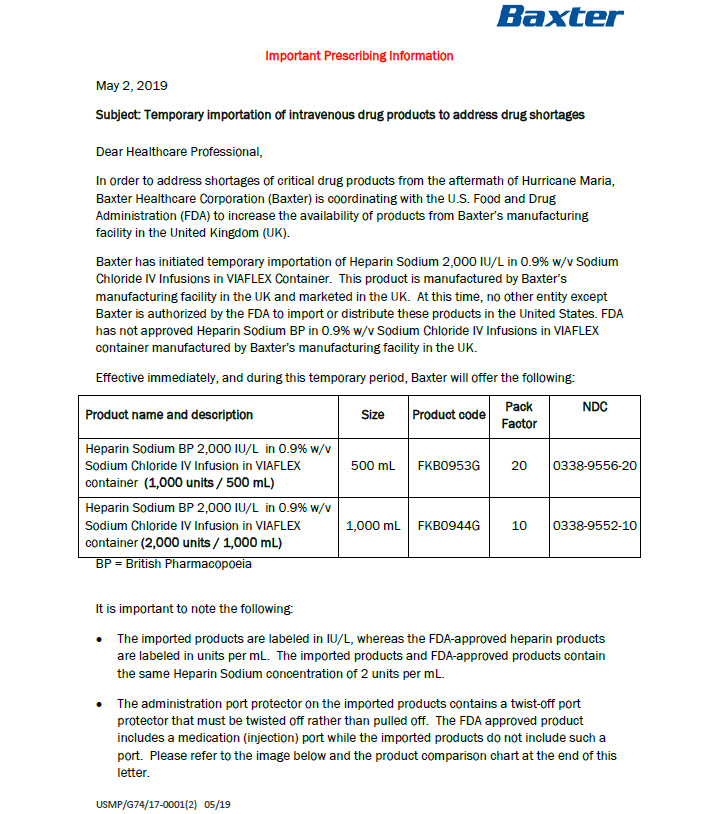
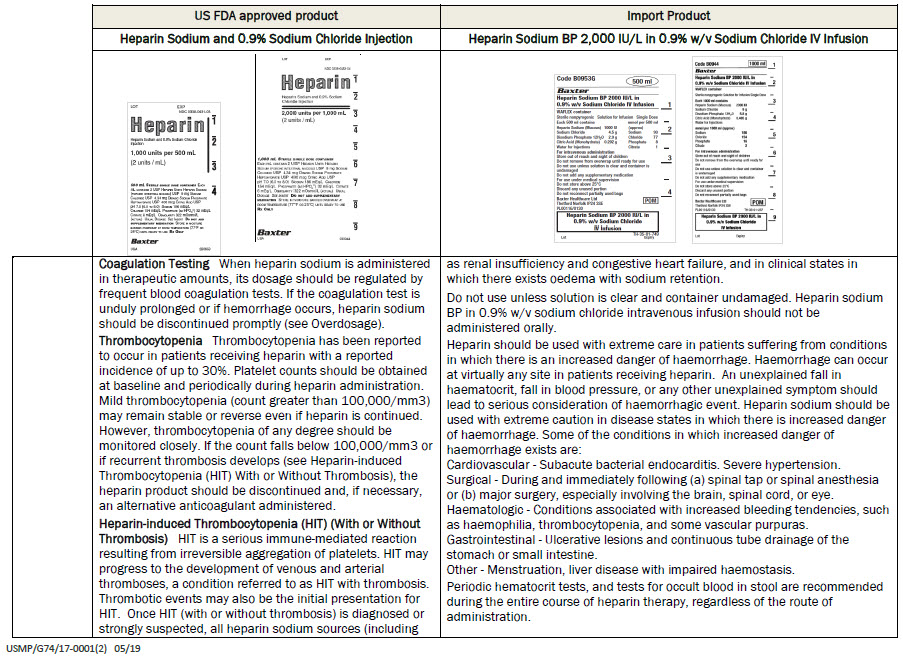
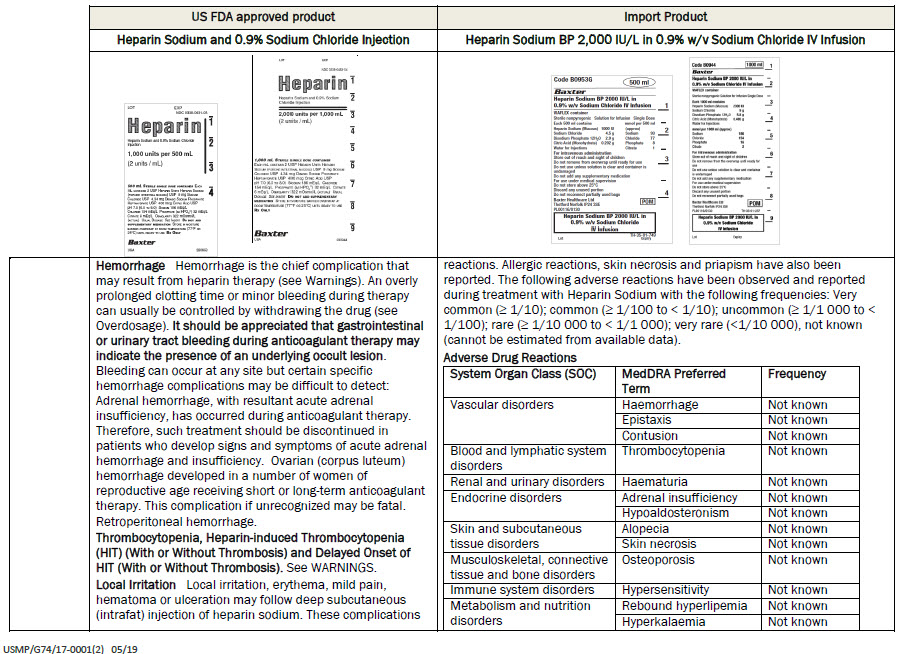
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
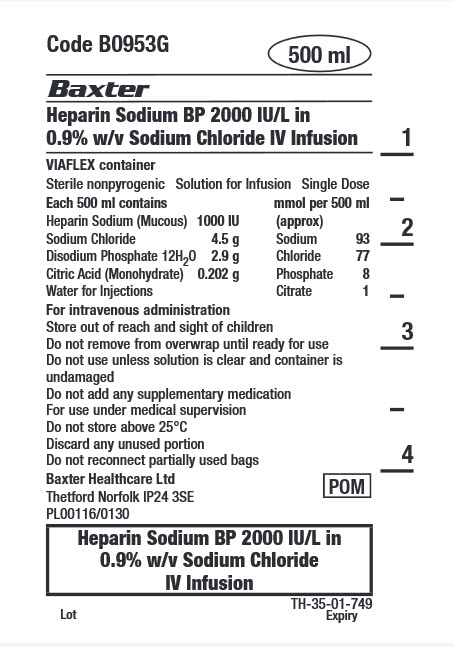
Code B0953G
500 ml
Baxter Logo
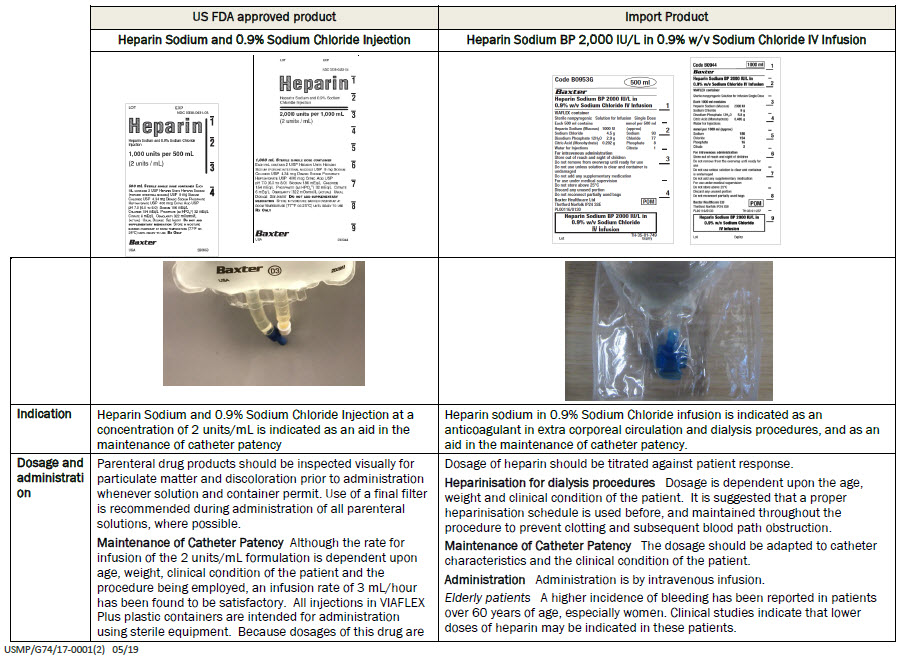
Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride IV InfusionVIAFLEX container
Sterile nonpyrogenic
Solution for Infusion
Single DoseEach 500 ml contains
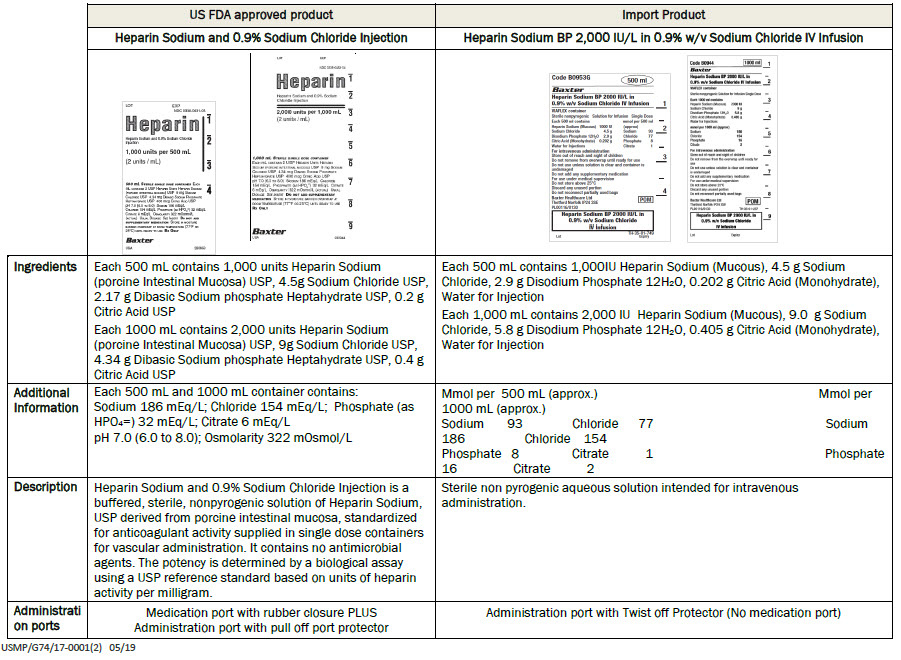
Heparin Sodium (Mucous) 1000 IU
Sodium Chloride 4.5 g
Disodium Phosphate 12H 2 0 2.9 g
Citric Acid (Monohydrate) 0.202g
Water for Injectionsmmol per 500 ml (approx)
Sodium 93
Chloride 77
Phosphate 8
Citrate 1For intravenous administration
Store out of reach and sight of children
Do not remove from overwrap until ready for use
Do not use unless solution is clear and container is
undamaged
Do not add any supplementary medication
For use under medical supervision
Do not store above 25°C
Discard any unused portion
Do not reconnect partially used bags
Baxter Healthcare Ltd
Thetford Norfolk IP24 3SE
PL00116/0130
POM symbol
Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride
IV Infusion
TH-35-01-749
Lot
Expiry
1
2
3
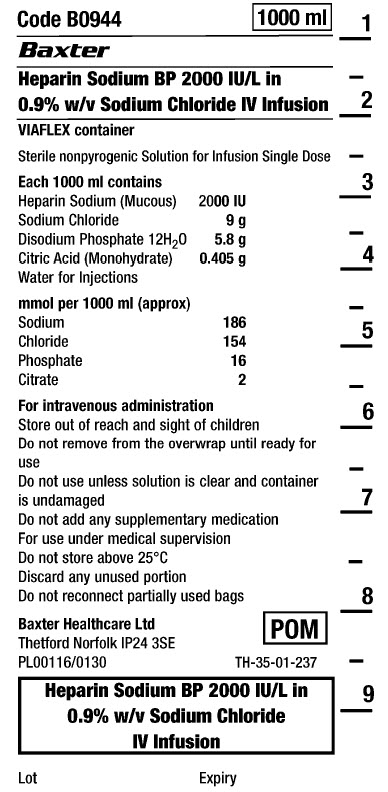
4Code B0944
1000 ml
Baxter Logo
Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride IV InfusionVIAFLEX container
Sterile nonpyrogenic
Solution for Infusion
Single Dose-
Each 1000 ml contains
Heparin Sodium (Mucous) 2000 IU
Sodium Chloride 9 g
Disodium Phosphate 12H 2 0 5.8 g
Citric Acid (Monohydrate) 0.405 g
Water for Injections
mmol per 1000 ml (approx)
Sodium 186
Chloride 154
Phosphate 16
Citrate 2For intravenous administration
Store out of reach and sight of children
Do not remove from overwrap until ready for
use
Do not use unless solution is clear and container
is undamaged
Do not add any supplementary medication
For use under medical supervision
Do not store above 25°C
Discard any unused portion
Do not reconnect partially used bagsBaxter Healthcare Ltd
Thetford Norfolk IP24 3SE
PL00116/0130
POM symbol
TH-35-01-237Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride
IV Infusion
Lot
Expiry
1
2
3
4
5
6
7
8
9 -
Each 1000 ml contains
-
INGREDIENTS AND APPEARANCE
HEPARIN SODIUM AND SODIUM CHLORIDE
heparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9556 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPARIN SODIUM (UNII: ZZ45AB24CA) (HEPARIN - UNII:T2410KM04A) HEPARIN 1000 [iU] in 500 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 4.5 g in 500 mL SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 2.9 g in 500 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.202 g in 500 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9556-20 20 in 1 CARTON 10/25/2017 1 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/25/2017 HEPARIN SODIUM AND SODIUM CHLORIDE
heparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9552 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPARIN SODIUM (UNII: ZZ45AB24CA) (HEPARIN - UNII:T2410KM04A) HEPARIN 2000 [iU] in 1000 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 g in 1000 mL SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 5.8 g in 1000 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.405 g in 1000 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9552-10 10 in 1 CARTON 10/25/2017 1 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/25/2017 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Ltd 221478644 ANALYSIS(0338-9556, 0338-9552) , MANUFACTURE(0338-9556, 0338-9552) , LABEL(0338-9556, 0338-9552) , PACK(0338-9556, 0338-9552) , STERILIZE(0338-9556, 0338-9552)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.