SENNA- sennosides liquid
Senna by
Drug Labeling and Warnings
Senna by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredient (per teaspoonful - 5 mL) Purpose
- Uses
-
Warnings
Do not use
laxative products for longer than 1 week unless directed by a doctor.
Ask a doctor or pharmacist before use if you have
stomach pain nausea vomiting
noticed a change in bowel movement that continues over a period of 2 weeks -
Directions
shake well before using Do not exceed recommended dose
Age Starting Dose Maximum Dose adults and children 12 years
and older2 to 3 teaspoonfuls once a day preferably at bedtime; increase as needed or as recommended by a doctor 3 teaspoons in the morning and 3 teaspoons at bedtime Under 12 years of age ask a doctor ask a doctor
-
Other Information
- store at room temperature 20° to 25°C (68° to 77°F).
- a brown, natural flavored syrup supplied in the following oral dosage form:
NDC: 0904-7528-41: 5 mL unit dose cup, in a tray of ten cups.
NDC: 0904-7528-94: Case contains 40 unit dose cups of 5 mL (0904-7528-41) packaged in 4 trays of 10 unit dose cups each. - Inactive ingredients:
- Questions or comments?
- Distributed by:
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SENNA
sennosides liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-7528 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.8 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-7528-94 4 in 1 CASE 03/06/2025 1 10 in 1 TRAY 1 NDC: 0904-7528-41 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 03/06/2025 Labeler - Major Pharmaceuticals (191427277)
Trademark Results [Senna]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SENNA 97868919 not registered Live/Pending |
Natty Collection LLC 2023-04-02 |
 SENNA 90583748 not registered Live/Pending |
Jeremey Decena 2021-03-17 |
 SENNA 90399285 not registered Live/Pending |
AYRTON SENNA EMPREENDIMENTOS LTDA. 2020-12-21 |
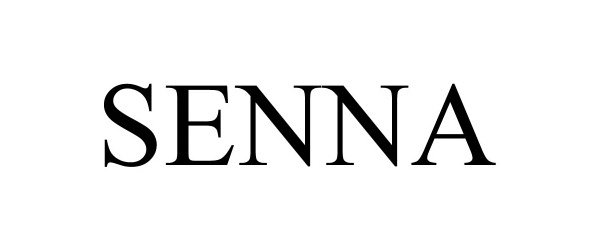 SENNA 90022160 not registered Live/Pending |
OPWEST DEVELOPMENT LLC 2020-06-26 |
 SENNA 88624114 not registered Live/Pending |
Ceritas Wines LLC 2019-09-19 |
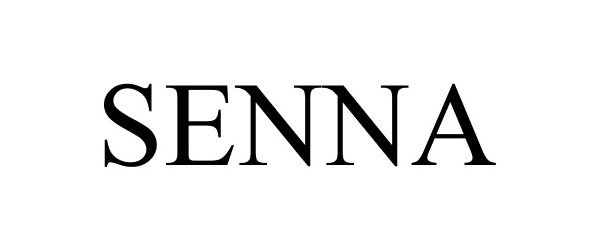 SENNA 87683504 5564030 Live/Registered |
OMM Imports Inc. 2017-11-14 |
 SENNA 76601884 3268781 Dead/Cancelled |
Studio RTA 2004-07-12 |
 SENNA 75170094 2188775 Live/Registered |
Senna Cosmetics, Inc. 1996-09-23 |
 SENNA 74561186 not registered Dead/Abandoned |
Senna Cosmetics, Inc. 1994-08-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
