DoloEar by Pharmadel LLC DoloEar (Hcare)
DoloEar by
Drug Labeling and Warnings
DoloEar by is a Homeopathic medication manufactured, distributed, or labeled by Pharmadel LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
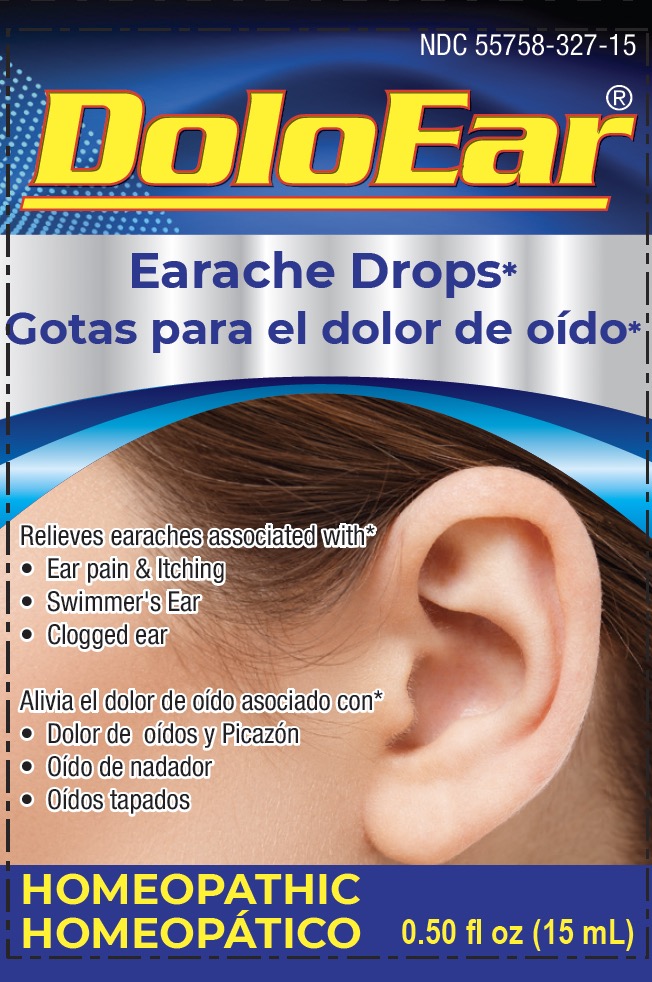
DOLOEAR- apis melifica, aristolochia clematitis, belladonna, chamomilla, lachesis mutus, thuja occidentalis solution/ drops
Pharmadel LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
DoloEar (Hcare)
Active ingredients & Purposes
| Active Ingredients** (HPUS) | Purposes* |
|---|---|
| Apis melifica 6C ....................... |
Ear pain, inflammation |
| Aristolochia clematitis 6C......... | Ear inflammation |
| Belladonna 6C.......................... | Throbbing pain |
| Chamomilla vulgaris 6C .......... |
Ear pain irritability |
| Lachesis mutus 6C .................. | Ear inflammation |
| Thuja occidentalis 6C .............. | Earache from pressure |
The letters HPUS indicate that this ingredient is officially included in the Homeopathic Pharmacopeia of the United States.
Uses*
Temporarily relieves earache symptoms associated with
- common pain and itching of swimmer's ear
- clogged ears
- ear pain or itching from other earaches after diagnosis by a doctor
Warnings
For use in ears only.
Directions
- shake before use
adults and children ages 6 and older:
- tilt head sideways and apply 2-3 drops into the ear (tip of dropper should not enter the ear canal)
- keep head tilted and allow drops to remain in the ear for 2 minutes or gently place cotton in ear to keep drops in
- repeat as needed, up to 4 times a day
children under 6 years of age: do not use
| DOLOEAR
apis melifica, aristolochia clematitis, belladonna, chamomilla, lachesis mutus, thuja occidentalis solution/ drops |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Pharmadel LLC (030129680) |
Trademark Results [DoloEar]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOLOEAR 86882244 5035565 Live/Registered |
Pharmadel LLC 2016-01-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.