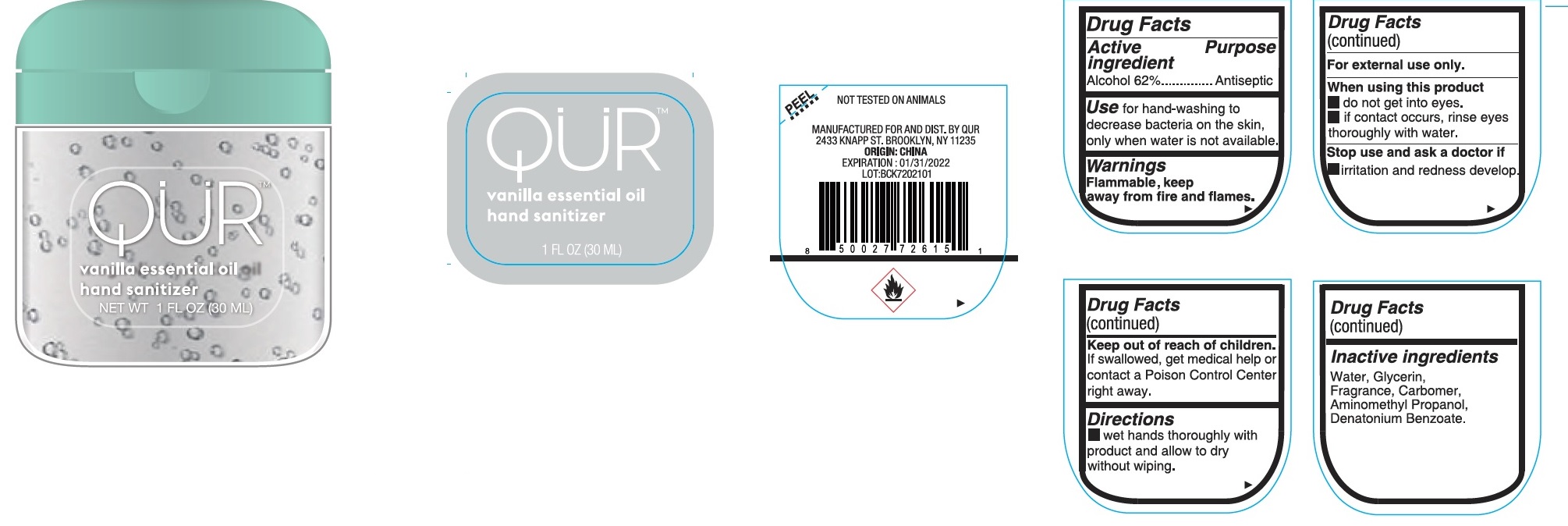
QUR Vanilla essential oil hand sanitizer
QUR Vanilla essential oil hand sanitizer by
Drug Labeling and Warnings
QUR Vanilla essential oil hand sanitizer by is a Otc medication manufactured, distributed, or labeled by Bath Concept Cosmetics (Dongguan) Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
QUR VANILLA ESSENTIAL OIL HAND SANITIZER- alcohol gel
Bath Concept Cosmetics (Dongguan) Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
QUR Vanilla essential oil hand sanitizer
| QUR VANILLA ESSENTIAL OIL HAND SANITIZER
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bath Concept Cosmetics (Dongguan) Co., Ltd (529623933) |
Revised: 4/2021
Document Id: bee93ee1-770a-ebaa-e053-2a95a90a9b4a
Set id: cfbfece6-867a-4cc6-9eb9-ba108722790e
Version: 2
Effective Time: 20210401
Ba
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.