Pimobendan by Medisca Inc Pimobendan USP
Pimobendan by
Drug Labeling and Warnings
Pimobendan by is a Other medication manufactured, distributed, or labeled by Medisca Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PIMOBENDAN- pimobendan powder
Medisca Inc
----------
Pimobendan USP
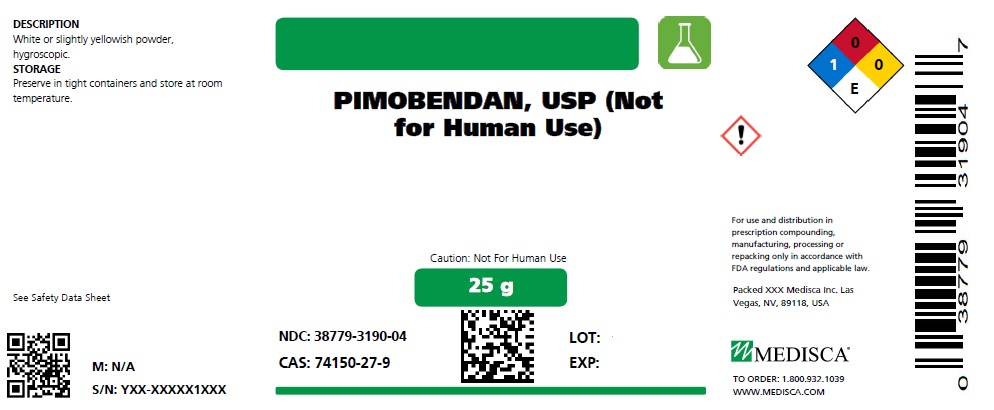
25 g
NDC: 38779-3190-04
See Safety Data Sheet
For use and distribution in prescription compounding, manufacturing, processing or repacking only in accordance with FDA regulations and applicable law.
DESCRIPTION
White or slightly yellowish powder, hygroscopic.
STORAGE
Preserve in tight containers and store at room temperature.
S/N: YXX-XXXXX1XXX
CAS: 74150-27-9
LOT:
EXP:
PIMOBENDAN, USP (Not
for Human Use)
Packed XXX Medisca Inc. Las Vegas, NV, 89118, USA
TO ORDER:1.800.9323.1039
WWW.MEDISCA.COM
M: N/A
Caution: Not For Human Use
| PIMOBENDAN
pimobendan powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Medisca Inc (794301960) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.