DOMEBORO- aluminum acetate gel
DOMEBORO by
Drug Labeling and Warnings
DOMEBORO by is a Otc medication manufactured, distributed, or labeled by Advantice Health, LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments
- SPL UNCLASSIFIED SECTION
-
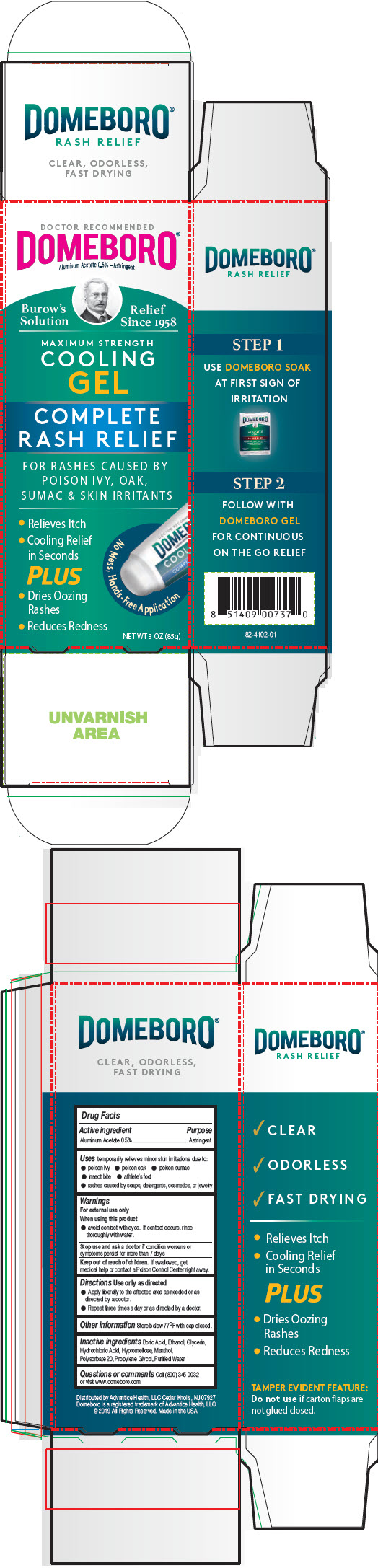
PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
DOCTOR RECOMMENDED
DOMEBORO®
Aluminum Acetate 0.5% - AstringentBurrow's
SolutionRelief
Since 1958MAXIMUM STRENGTH
COOLING
GELCOMPLETE
RASH RELIEFFOR RASHES CAUSED BY
POISON IVY, OAK,
SUMAC & SKIN IRRITANTS- Relieves Itch
- Cooling Relief
in Seconds
PLUS - Dries Oozing
Rashes - Reduces Redness
No Mess, Hands-Free Application
NET WT 3 OZ (85g)
-
INGREDIENTS AND APPEARANCE
DOMEBORO
aluminum acetate gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 16864-250 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ACETATE (UNII: 80EHD8I43D) (ALUMINUM CATION - UNII:3XHB1D032B) ALUMINUM ACETATE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) HYDROCHLORIC ACID (UNII: QTT17582CB) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16864-250-01 1 in 1 CARTON 04/15/2020 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 04/15/2020 Labeler - Advantice Health, LLC. (192527062)
Trademark Results [DOMEBORO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOMEBORO 71564320 0515190 Live/Registered |
DOME CHEMICALS, INC. 1948-08-28 |
 DOMEBORO 71433813 0382555 Dead/Cancelled |
DOME CHEMICALS, INC. 1940-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.