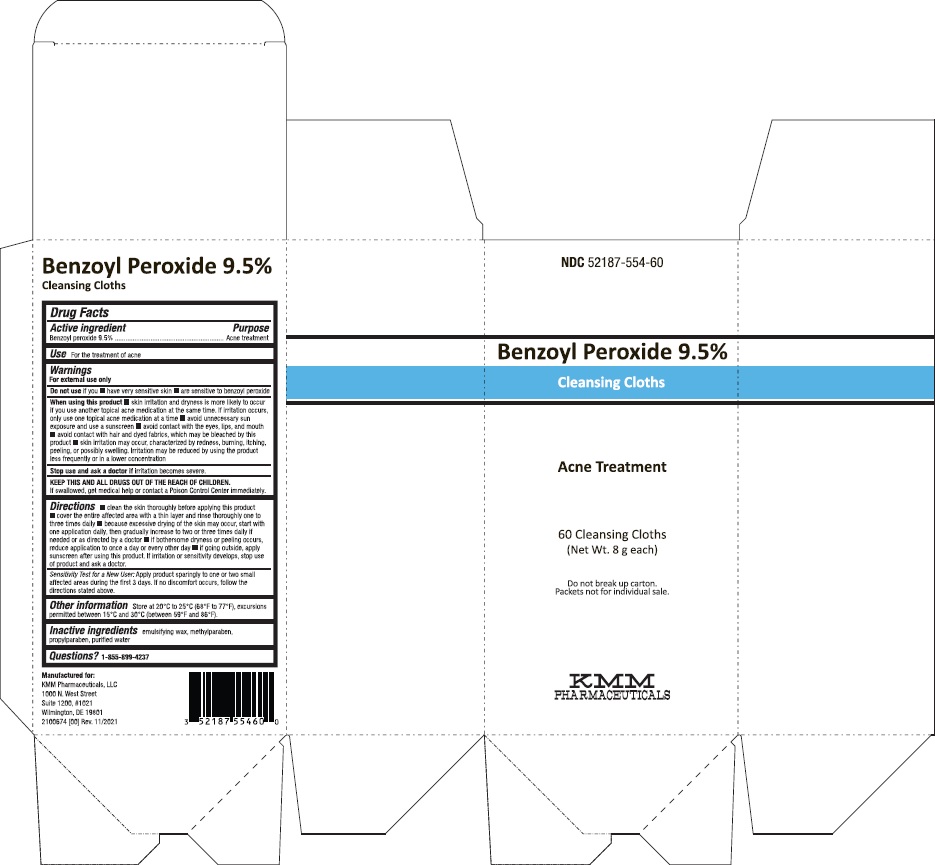
Benzoyl Peroxide 9.5% Cleansing Cloths
BENZOYL PEROXIDE by
Drug Labeling and Warnings
BENZOYL PEROXIDE by is a Otc medication manufactured, distributed, or labeled by KMM Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BENZOYL PEROXIDE- benzoyl peroxide cloth
KMM Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Benzoyl Peroxide 9.5% Cleansing Cloths
Warnings
For external use only
Do not use
if you
- have very sensitive skin
- are sensitive to benzoyl peroxide
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
Stop use and ask a doctor if irritation becomes severe.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
| BENZOYL PEROXIDE
benzoyl peroxide cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - KMM Pharmaceuticals, LLC (078521761) |