Synanthic® oxfendazole oral suspension Bovine Dewormer Suspension, 9.06%
Synanthic Bovine Dewormer by
Drug Labeling and Warnings
Synanthic Bovine Dewormer by is a Animal medication manufactured, distributed, or labeled by Boehringer Ingelheim Animal Health USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SYNANTHIC BOVINE DEWORMER- oxfendazole suspension
Boehringer Ingelheim Animal Health USA Inc.
----------
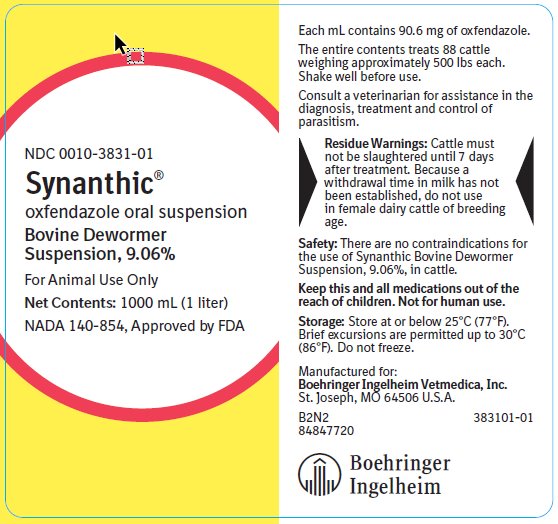
Synanthic®
oxfendazole oral suspension
Bovine Dewormer Suspension, 9.06%
The entire contents of the 1 liter bottle treats 88 cattle weighing approximately 500 lbs each.
The entire contents of the 4 liter bottle treats 352 cattle weighing approximately 500 lbs each.
Shake well before use.
Safety:
There are no contraindications for the use of Synanthic Bovine Dewormer Suspension, 9.06%, in cattle.
Storage:
Store at or below 25°C (77°F). Brief excursions are permitted up to 30°C (86°F). Do not freeze.
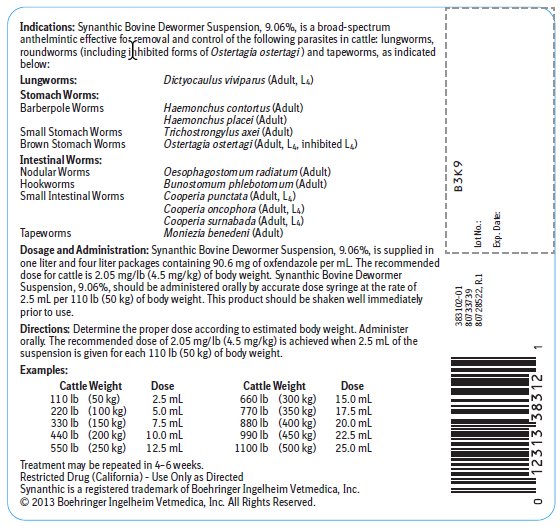
Indications:
Synanthic Bovine Dewormer Suspension, 9.06%, is a broad-spectrum anthelmintic effective for removal and control of the following parasites in cattle: lungworms, roundworms (including inhibited forms of Ostertagia ostertagi ) and tapeworms, as indicated below:
|
Lungworms: |
Dictyocaulus viviparus (Adult, L4) |
|
Stomach Worms: | |
|
Barberpole Worms |
Haemonchus contortus (Adult) |
|
Haemonchus placei (Adult) |
|
|
Small Stomach Worms |
Trichostrongylus axei (Adult) |
|
Brown Stomach Worms |
Ostertagia ostertagi (Adult, L4, inhibited L4) |
|
Intestinal Worms: | |
|
Nodular Worms |
Oesophagostomum radiatum (Adult) |
|
Hookworms |
Bunostomum phlebotomum (Adult) |
|
Small Intestinal Worms |
Cooperia punctata (Adult, L4) |
|
Cooperia oncophora (Adult, L4) |
|
|
Cooperia surnabada (Adult, L4) |
|
|
Tapeworms |
Moniezia benedeni (Adult) |
Dosage and Administration:
Synanthic Bovine Dewormer Suspension, 9.06%, is supplied in one liter and four liter packages containing 90.6 mg of oxfendazole per mL. The recommended dose for cattle is 2.05 mg/lb (4.5 mg/kg) of body weight. Synanthic Bovine Dewormer Suspension, 9.06%, should be administered orally by accurate dose syringe at the rate of 2.5 mL per 110 lb (50 kg) of body weight. This product should be shaken well immediately prior to use.
Directions:
Determine the proper dose according to estimated body weight. Administer orally. The recommended dose of 2.05 mg/lb (4.5 mg/kg) is achieved when 2.5 mL of the suspension is given for each 110 lb (50 kg) of body weight.
Examples:
|
Cattle Weight |
Dose |
Cattle Weight |
Dose |
|||
|
110 lb |
(50 kg) |
2.5 mL |
660 lb |
(300 kg) |
15.0 mL |
|
|
220 lb |
(100 kg) |
5.0 mL |
770 lb |
(350 kg) |
17.5 mL |
|
|
330 lb |
(150 kg) |
7.5 mL |
880 lb |
(400 kg) |
20.0 mL |
|
|
440 lb |
(200 kg) |
10.0 mL |
990 lb |
(450 kg) |
22.5 mL |
|
|
550 lb |
(250 kg) |
12.5 mL |
1100 lb |
(500 kg) |
25.0 mL |
|
Treatment may be repeated in 4–6 weeks.
Restricted Drug (California) - Use Only as Directed
Synanthic is a registered trademark of Boehringer Ingelheim Vetmedica, Inc.
© 2013 Boehringer Ingelheim Vetmedica, Inc. All Rights Reserved.
Manufactured for:
Boehringer Ingelheim Vetmedica, Inc.
St. Joseph, MO 64506 U.S.A.
B2N2 84847720 383101-01
383102-01 80733739 80728522, R.1
Principal Display Panel – 1 L Front Label
NDC: 0010-3831-01
Synanthic®
oxfendazole oral suspension
Bovine Dewormer Suspension, 9.06%
For Animal Use Only
Net Contents: 1000 mL (1 liter)
NADA 140-854, Approved by FDA
| SYNANTHIC BOVINE DEWORMER
oxfendazole suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.