WILATE - VON WILLEBRAND FACTOR/COAGULATION FACTOR VIII COMPLEX (HUMAN) (von willebrand factor/coagulation factor viii complex- human powder, for solution
Wilate - von Willebrand Factor/Coagulation Factor VIII Complex (Human) by
Drug Labeling and Warnings
Wilate - von Willebrand Factor/Coagulation Factor VIII Complex (Human) by is a Other medication manufactured, distributed, or labeled by Octapharma Pharmazeutika Produktionsgesellschaft m.b.H.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Wilate safely and effectively. See full prescribing information for Wilate.
Wilate, von Willebrand Factor/Coagulation Factor VIII Complex (Human), Powder for Solution, for Intravenous Use Only.
Initial U.S. Approval: 2009INDICATIONS AND USAGE
Wilate is a von Willebrand Factor/Coagulation Factor VIII Complex (Human) indicated for the treatment of spontaneous and trauma-induced bleeding episodes in patients with severe von Willebrand disease (VWD) as well as patients with mild or moderate VWD in whom the use of desmopressin is known or suspected to be ineffective or contraindicated. ( 1 )
Wilate is not indicated for the prophylaxis of spontaneous bleeding episodes, or the prevention of excessive bleeding during and after surgery in VWD patients.
Wilate is not indicated for Hemophilia A
DOSAGE AND ADMINISTRATION
For Intravenous Use Only (2)
(2)
Type of Hemorrhages Loading Dosage (IU VWF:RCo /kg BW) Maintenance Dosage (IU VWF:RCo /kg BW) Therapeutic Goal Minor (2)
20-40 IU/kg (2)
20-30 IU/kg every 12-24 hours (2)
VWF:RCo and FVIII activity through levels of >30% (2)
Major (2)
40-60 IU/kg (2)
20-40 IU/kg every 12-24 hours (2)
VWF:RCo and FVIII activity through levels of >50% (2)
(2)
DOSAGE FORMS AND STRENGTHS
Wilate is a sterile, lyophilized powder for reconstitution for intravenous injection, provided in the following nominal strengths per vial ( 3 ):
500 IU VWF:RCo and 500 IU FVIII activities in 5 mL
1000 IU VWF:RCo and 1000 IU FVIII activities in 10 mL
CONTRAINDICATIONS
Hypersensitivity with known anaphylactic or severe systemic reaction to human plasma-derived products, any ingredient in the formulation, or components of the container. ( 4 )
WARNINGS AND PRECAUTIONS
Hypersensitivity reaction ( 5.1 )
Thromboembolic events associated with von Willebrand factor/Coagulation Factor FVIII (VWF/FVIII) products: plasma levels of FVIII activity should be monitored to avoid sustained excessive FVIII levels, which may increase the risk of thrombotic events ( 5.2 )
Potential for inducing antibodies to Factor VIII (inhibitors) and antibodies to VWF, especially in VWD type 3 patients ( 5.3 )
Theoretical risk of infectious agents transmission as the product is made from human plasma ( 5.4 )
ADVERSE REACTIONS
The most common adverse reactions in clinical studies on VWD were urticaria and dizziness (each 2.2%) ( 6.1 ).
To report SUSPECTED ADVERSE REACTIONS, contact Octapharma USA Inc. at phone # 866-766-4860 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
None known ( 7 ).
USE IN SPECIFIC POPULATIONS
Pregnancy: No human or animal data. Use only if clearly needed ( 8.1 ).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: August 2010
_____________________________________________
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in von Willebrand Disease
2.2 Dosing Schedule
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Thromboembolic Risk
5.3 Inhibitor Formation
5.4 Infection Risk from Human Plasma
5.5 Monitoring and Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Wilate is a von Willebrand Factor/Coagulation Factor VIII Complex (Human) indicated for the treatment of spontaneous and trauma-induced bleeding episodes in patients with severe von Willebrand disease (VWD) as well as patients with mild or moderate VWD in whom the use of desmopressin is known or suspected to be ineffective or contraindicated.
Clinical trials to evaluate the safety and efficacy of prophylactic dosing with Wilate to prevent spontaneous bleeding have not been conducted in VWD subjects.
Wilate is not indicated for the prevention of excessive bleeding during and after surgery in VWD patients.
Wilate is not indicated for Hemophilia A.
-
2 DOSAGE AND ADMINISTRATION
- For Intravenous Use after Reconstitution
- Treatment should be initiated under the supervision of a physician experienced in the treatment of coagulation disorders.
- Each vial of Wilate contains the labeled amount in International Units (IU) of von Willebrand factor (VWF) activity as measured with the Ristocetin cofactor assay (VWF:RCo), and coagulation factor VIII (FVIII) activity measured with the chromogenic substrate assay.
- The number of units of VWF:RCo and FVIII activities administered is expressed in IU, which are related to the current WHO standards for VWF and FVIII products. VWF:RCo and FVIII activities in plasma are expressed either as a percentage (relative to normal human plasma) or in IU (relative to the International Standards for VWF:RCo and FVIII activities in plasma).
2.1 Dosage in von Willebrand Disease
The ratio between VWF:RCo and FVIII activities in Wilate is approximately 1:1.
The dosage should be adjusted according to the extent and location of the bleeding. In VWD type 3 patients, especially in those with gastro-intestinal (GI) bleedings, higher doses may be required.
2.2 Dosing Schedule
Physician supervision of the treatment regimen is required. A guide for dosing in the treatment of major and minor hemorrhages is provided in Table 1 .
The careful control of replacement therapy is especially important in life-threatening hemorrhages. When using a FVIII-containing VWF product, the treating physician should be aware that continued treatment may cause an excessive rise in FVIII activity.[ 1 ]
Table 1 Guide to Wilate Dosing for Treatment of Minor and Major Hemorrhages
Type of Hemorrhages Loading Dosage (IU VWF:RCo /kg BW) Maintenance Dosage (IU VWF:RCo /kg BW) Therapeutic Goal Minor Hemorrhages
20-40 IU/kg
20-30 IU/kg every 12-24 hours*
VWF:RCo and FVIII activity through levels of >30%
Major Hemorrhages
40-60 IU/kg
20-40 IU/kg every 12-24 hours*
VWF:RCo and FVIII activity through levels of >50%
*This may need to be continued for up to 3 days for minor hemorrhages and 5-7 days for major hemorrhages
Repeat doses are administered for as long as needed based upon repeat monitoring of appropriate clinical and laboratory measures.
Although dose can be estimated by the guidelines above, it is highly recommended that whenever possible, appropriate laboratory tests should be performed on the patient’s plasma at suitable intervals to assure that adequate VWF:RCo and FVIII activity levels have been reached and are maintained.
In the unlikely event that a patient who is actively bleeding should miss a dose, it may be appropriate to adopt a dosage depending on the level of coagulation factors measured, extent of the bleeding, and patient's clinical condition.
2.3 Administration
Wilate is administered via intravenous infusion. Wilate is provided with a Mix2Vial TM transfer device for reconstitution of the freeze-dried powder in diluent, a 10-mL syringe, an infusion set and two alcohol swabs.
Instructions for Reconstitution:
1) Warm the Powder and Diluent in the closed vials up to room temperature. This temperature should be maintained during reconstitution. If a water bath is used for warming, care must be taken to avoid water coming into contact with the rubber stoppers (latex-free) or the caps of the vials. The temperature of the water bath should not exceed +37°C (98°F).
2) Remove the caps from the concentrate (Wilate) vial and the diluent vial and clean the rubber stoppers with an alcohol swab.

3) Peel away the lid of the outer package of the Mix2Vial™ transfer set. To maintain sterility, leave the Mix2Vial™ device in the clear outer packaging. Place the diluent vial on a level surface and hold the vial firmly. Take the Mix2Vial™ in its outer package and invert it over the diluent vial. Push the blue plastic cannula of the Mix2Vial™ firmly through the rubber stopper of the diluent vial (Fig. 1). While holding onto the diluent vial, carefully remove the outer package from the Mix2Vial™, being careful to leave the Mix2Vial™ attached firmly to the diluent vial (Fig. 2).
4) With the concentrate (Wilate) vial held firmly on a level surface, quickly invert the diluent vial with the Mix2Vial™ attached and push the transparent plastic cannula end of the Mix2Vial™ firmly through the stopper of the concentrate (Wilate) vial (Fig. 3). The diluent will be drawn into the concentrate (Wilate) vial by the vacuum.
5) With both vials still attached, gently swirl the product vial to ensure the product is fully dissolved to a clear solution. Once the contents of the Wilate vial are completely dissolved, firmly hold both the transparent and blue parts of the Mix2Vial™. Unscrew the Mix2Vial™ into two separate pieces (Fig. 4) and discard the empty diluent vial and the blue part of the Mix2Vial™.
- The powder should be reconstituted only directly before injection. As Wilate contains no preservatives, the solution should be used immediately after reconstitution.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- The filtered solution is clear or slightly opalescent, colourless or slightly yellow. If the concentrate fails to dissolve completely or an aggregate is formed, the preparation must not be used.
Instructions for Injection:
- With the Wilate vial still upright, attach a plastic disposable syringe to the Mix2Vial™ (transparent plastic part). Invert the system and draw the reconstituted Wilate into the syringe.
- Once Wilate has been transferred into the syringe, firmly hold the barrel of the syringe (keeping the syringe plunger facing down) and detach the Mix2Vial™ from the syringe. Discard the Mix2Vial™ (transparent plastic part) and empty Wilate vial.
- Clean the intended injection site with an alcohol swab.
- Attach a suitable infusion needle to the syringe.
- Inject the solution intravenously at a slow speed of 2-4 mL/minute.
- As a precautionary measure, the patient’s pulse rate should be measured before and during the injection. If a marked increase in the pulse rate occurs, the injection speed must be reduced or the administration must be interrupted.
- Any unused product or waste material should be disposed of in accordance with local requirements.
Incompatibilities
Wilate must not be mixed with other medicinal products or administered simultaneously with other intravenous preparation in the same infusion set.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Wilate is contraindicated for patients who have known anaphylactic or severe systemic reaction to plasma-derived products, any ingredient in the formulation, or components of the container. For a complete listing of ingredients, see Description ( 11 ).
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Hypersensitivity or allergic reactions (which may include angioedema, burning and stinging at the infusion site, chills, flushing, generalized urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing) have been observed upon use of Wilate, and may in some cases progress to severe anaphylaxis (including shock) with or without fever.[ 2 ] Closely monitor patients receiving Wilate and carefully observe for any symptoms throughout the infusion period.
Inform patients of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If allergic symptoms occur, discontinue the administration immediately and contact the physician. Since inhibitor antibodies may occur concomitantly with anaphylactic reactions, patients experiencing an anaphylactic reaction should also be evaluated for the presence of inhibitors.[ 2 ]
5.2 Thromboembolic Risk
When using a FVIII-containing VWF product, the treating physician should be aware that continued treatment may cause an excessive rise in FVIII activity.[ 1 ] Monitor plasma levels of VWF:RCo and FVIII activities in patients receiving Wilate to avoid sustained excessive VWF and FVIII activity levels, which may increase the risk of thrombotic events.
5.3 Inhibitor Formation
Patients with VWD, especially type 3 patients, may potentially develop neutralizing antibodies (inhibitors) to VWF. If a patient develops inhibitor to VWF (or FVIII), the condition will manifest itself as an inadequate clinical response. Thus, if the expected VWF activity plasma levels are not attained, or if bleeding is not controlled with an adequate dose or repeated dosing, perform an appropriate assay to determine if a VWF inhibitor is present. In patients with antibodies against VWF, VWF is not effective and infusion of this protein may lead to severe adverse events. Consider other therapeutic options for such patients. Physicians with experience in the care of patients with hemostatic disorders should direct their management.[ 3 ] In all such cases, it is recommended that a center specialized in bleeding disorders be contacted.
Since inhibitor antibodies may occur concomitantly with anaphylactic reactions, patients experiencing an anaphylactic reaction should also be evaluated for the presence of inhibitors.[ 2 ]
5.4 Infection Risk from Human Plasma
Wilate is made from human plasma. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the variant Creutzfeldt-Jakob disease (vCJD) agent. There is also the possibility that unknown infectious agents may be present in such products. The risk that such products will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, such products may still potentially transmit disease. [ 4 ]
Record the batch number of the product every time Wilate is administered to a patient, and consider appropriate vaccination (against hepatitis A and B virus) of patients in regular/repeated receipt of Wilate. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Octapharma USA, Inc., telephone # 1-866-766-4860.
5.5 Monitoring and Laboratory Tests
Monitor plasma levels of VWF:RCo and FVIII activities in patients receiving Wilate to avoid sustained excessive VWF and FVIII activity levels, which may increase the risk of thrombotic events, particularly in patients with known clinical or laboratory risk factors.
Monitor for development of VWF and FVIII inhibitors. Perform assays to determine if VWF and/or FVIII inhibitor(s) is present if bleeding is not controlled with the expected dose of Wilate. [ 5 ]
-
6 ADVERSE REACTIONS
The most common adverse reactions to treatment with Wilate in patients with VWD have been urticaria and dizziness.
The most serious adverse reactions to treatment with Wilate in patients with VWD have been hypersensitivity reactions.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trails of another drug and may not reflect the rates observed in clinical practice.
There were 92 VWD patients who received Wilate on 5676 occasions including clinical studies that involved prophylactic use, treatment on demand, surgery, and pharmacokinetics. Their safety data showed that the most common adverse reactions were urticaria and dizziness (each with 2 patients; 2.2%). There were also four patients (4.4%) who showed seroconversion for antibodies to parvovirus B19 not accompanied by clinical signs of disease. Seroconversion has not been reported since implementation of minipool testing of plasma used for the manufacture of Wilate.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during the post approval use of Wilate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
Post-marketing adverse reactions reported in patients treated for VWD include hypersensitivity reactions, dyspnea, nausea, vomiting, and cough.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with Wilate. It is also not known whether Wilate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Wilate should be given to a pregnant woman only if clearly needed.
8.2 Labor and Delivery
Wilate has not been studied in labor or delivery. It should be administered to VWF-deficient women at labor or delivery only if clearly indicated. [ 6 ]
8.4 Pediatric Use
Eleven pediatric patients with VWD between 5 to 16 years of age (8 type 3, 1 type 2, 2 type 1) were treated with Wilate for 234 bleeding episodes (BEs) in clinical studies. These studies showed that 88% of the BEs were treated successfully in this population ( Table 7 ). No dose adjustment is needed for pediatric patients as administered dosages were similar to those used in the adult population ( Table 8 ).
-
11 DESCRIPTION
Wilate is a human plasma-derived, sterile, purified, double virus inactivated von Willebrand Factor/Coagulation Factor VIII Complex (Human). Wilate is supplied as a lyophilized powder for reconstitution for intravenous injection.
Wilate is labeled with the actual VWF:RCo and FVIII activities in IU per vial. The VWF activity (VWF:RCo) is determined using a manual agglutination method referenced to the current “WHO International Standard for von Willebrand Factor Concentrate”. The FVIII activity is determined using a chromogenic substrate assay referenced to the current “WHO International Standard for Human Coagulation Factor VIII Concentrate”. The assay methodologies are according to European Pharmacopoeia (Ph.Eur.).
Wilate contains no preservative. The diluent for reconstitution of the lyophilized powder is Water for Injection with 0.1% Polysorbate 80.
No albumin is added as a stabilizer. The resulting specific activity of Wilate is ≥ 60 IU VWF:RCo and ≥ 60 IU FVIII activities per mg of total protein.
The nominal composition of Wilate is as follows:
Component Quantity/ 5 mL vial Quantity/ 10 mL vial VWF:RCo
500 IU
1000 IU
FVIII
500 IU
1000 IU
Total protein
≤ 7.5 mg
≤ 15.0 mg
Glycine
50 mg
100 mg
Sucrose
50 mg
100 mg
Sodium chloride
117 mg
234 mg
Sodium citrate
14.7 mg
29.4 mg
Calcium chloride
0.8 mg
1.5 mg
Water for injection
5 mL
10 mL
Polysorbate 80
1 mg/mL
1 mg/mL
Wilate is derived from large pools of human plasma collected in U.S. FDA approved plasma donation centers. All plasma donations are tested for viral markers in compliance with requirements of EU CPMP and FDA guidances. In addition, the limit for the titer of human parvovirus B19 DNA in the manufacturing pool is set not to exceed 10 4 IU/mL.
The product is manufactured from cryoprecipitate, which is reconstituted in a buffer and treated with aluminum hydroxide followed by two different chromatography steps, ultra- and diafiltration, and sterile filtration. The manufacturing process includes two virus inactivation steps, namely, treatment with an organic solvent/detergent (S/D) mixture, composed of tri-n-butyl phosphate (TNBP) and Octoxynol-9, and a terminal dry heat (TDH) treatment of the lyophilized product in final container [at +100°C (212°F) for 120 minutes at a specified residual moisture level of 0. 7 – 1.6%]. In addition, the ion-exchange chromatography step utilized during Wilate manufacturing also removes some viruses [ 7 ]. The mean cumulative virus reduction factors of these steps are summarized in Table 2 .
Table 2 Virus Reduction During Wilate Manufacturing
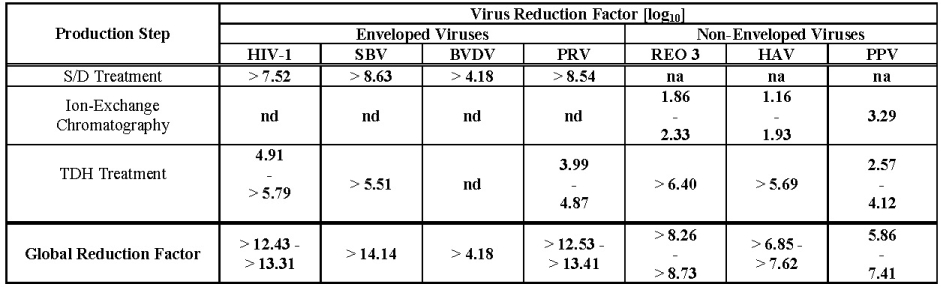
na: not applicable
nd: not done (S/D reagents present)
HIV-1: Human Immunodeficiency Virus - 1
SBV: Sindbis Virus
BVDV: Bovine Viral Diarrhea Virus
PRV: Pseudorabies Virus
REO 3: Reovirus Type 3
HAV: Hepatitis A Virus
PPV: Porcine Parvovirus
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VWF and FVIII are normal constituents of human plasma. VWF is a multimeric protein with two key functions. It is an adhesive molecule, which mediates the binding between platelets and damaged sub-endothelial tissues. It is also a carrier protein, involved in the transport and stabilization of FVIII. Patients suffering from VWD have a deficiency or abnormality of VWF. This reduction in VWF concentration in the bloodstream result in a correspondingly low FVIII activity and an abnormal platelet function thereby resulting in excessive bleeding. [ 8 ]
The VWF in Wilate is derived from normal human plasma and is expected to behave in the same way as endogenous VWF. Thus, administration of VWF allows correction of the hemostatic abnormalities in VWD patients at two levels:
- The VWF re-establishes platelet adhesion to the vascular sub-endothelium at the site of vascular damage (as it binds both to the vascular sub-endothelium and to the platelet membrane), providing primary hemostasis, as shown by the shortening of the bleeding time. This effect occurs immediately.
- The VWF induces correction of the associated FVIII deficiency in VWD. Administered intravenously, VWF binds endogenous FVIII (which is produced normally by the patient), and by stabilizing this factor, avoids its rapid degradation. This action is slightly delayed. However, administration of a FVIII-containing VWF preparation like Wilate rapidly restores the FVIII activity level to normal.[ 8 ]
12.3 Pharmacokinetics
An open label, prospective, randomized, controlled, two-arm cross-over Phase 2 study with Wilate and a comparator product was conducted at 6 sites in the US. In this study, pharmacokinetic (PK) profiles of Wilate were determined by FVIII activity, VWF:RCo, VWF:Ag, and VWF:CB.
Each of twenty-two subjects with inherited VWD [Type 1, n=6; Type 2, n=9 (6 Type 2A, 1 Type 2B, and 2 Type 2M); and Type 3, n=7] received an intravenous bolus dose of Wilate containing approximately 40 IU of VWF:RCo/kg BW. Twenty subjects completed the study as per protocol. PK parameters of VWF:RCo and FVIII are summarized in Table 3 and Table 4 , respectively.
Table 3 Pharmacokinetic Parameters of VWF:RCo:mean ± SD (range)
Parameters VWD type I (n = 5) VWD type II (n = 9) VWD type III (n = 6) Total (n = 20) Cmax (IU/dL)
74 ± 13
(62 - 91)
77 ± 18
(40 - 100)
79 ± 13
(65 - 102)
76 ± 15
(40 - 102)
AUC(0-inf)
(IU*hr/dL)
1633 ± 979
(984 - 3363)
1172 ± 421
(571 - 1897)
995 ± 292
(527 - 1306)
1235 ± 637
(527 - 3363)
Half-life (hrs)
24.7 ± 17.9
(11.2 - 48.5 )
15.3 ± 6.3
(6.0 - 26.4)
9.1± 2.6
(5.7 - 12.9 )
15.8 ± 11.0
(5.7 - 48.5)
CL (mL/h/kg)
3.1 ± 1.1
(1.2 - 4.1)
4.1 ± 1.7
(2.0 - 7.1)
4.2 ± 1.4
(3.0 - 6.6)
3.9 ± 1.5
(1.2 - 7.1)
Vss (mL/kg)
81.7 ± 38.5
(15.3 - 74.2)
76.6 ± 35.4
(45.3 - 158.8)
49.4 ± 16.7
(29.7 - 67.1)
69.7 ± 33.2
(29.7 - 158.8)
MRT (hrs)
32.7 ± 25.8
(15.3 - 74.2)
19.7 ± 5.6
(9.9 - 27.1)
11.9 ± 2.9
(9.2 - 15.9)
20.6 ± 14.8
(9.2 - 74.2)
Recovery
(%IU/kg)
1.8 ± 0.2
(1.5 - 2.0)
1.8 ± 0.5
(1.0 - 2.4)
2.1 ± 0.3
(1.8 - 2.6)
1.9 ± 0.4
(1.0 - 2.6)
C max = peak concentration; AUC = area under curve; CL = clearance; Vss = volume of distribution at steady state; MRT = mean residence time
The PK parameters reported in Table 3 are based on VWF:RCo values obtained using a modified Behring Coagulation System (BCS) analytical method. The modified BCS was used because of its validated lower variability compared to the standard BCS. The measured concentrations (IU VWF:RCo/mL) are higher by the modified BCS than by the standard BCS analytical method which is used in some clinical laboratories. Dose adjusted C max and AUC determined by this modified BCS method are approximately 1.5 times higher than those by the standard BCS method. No difference has been found in incremental recovery.
Table 4 Pharmacokinetic Parameters of FVIII:C: mean ± SD (range) - chromogenic
Parameters VWD type I (n = 5) VWD type II (n = 8*) VWD type III (n = 6) Total (n = 19*) Cmax (IU/dL)
117.1 ± 12.1
(103 - 135)
147.2 ± 32.6
(102 - 206)
120 ± 23
(91 - 148)
112 ± 23
(59 - 148)
AUC(0-inf)
(IU*hr/dL)
1187 ± 382
(523 - 1483)
1778 ± 1430
(544 - 4821)
2670 ± 854
(1874 - 3655)
2290 ± 1045
(464 - 4424)
Half-life (hrs)
17.5 ± 4.9
(10.9 - 23.8)
23.6 ± 8.3
(12.6 - 34.7)
16.1 ± 3.1
(11.8 - 20.1)
19.6 ± 6.9
(10.9 - 34.7)
CL (mL/h/kg)
4.4 ± 3.7
(2.5 - 11.0)
2.5 ± 0.9
(1.2 - 3.5)
2.0 ± 0.6
(1.4 - 2.8)
2.9 ± 2.1
(1.2 - 11.0)
Vss (mL/kg)
95.0 ± 53.8
(57.1 - 190.0)
79.5 ± 23.1
(52.8 - 116.2)
44.2 ± 10.4
(31.8 - 57.1)
72.4 ± 36.2
(31.8 - 190.0)
MRT (hrs)
24.1 ± 5.5(17.2 - 31.5)
35.1 ± 14.2
(17.5 - 61.6)
23.0 ± 3.7
(18.0 - 27.7)
28.4 ± 11.1
(17.2 - 61.6)
Recovery
(%IU/kg)
1.9 ± 0.5
(1.1 - 2.5)
2.2 ± 0.4
(1.6 - 2.8)
2.5 ± 0.5
(2.0 - 3.0)
2.2 ± 0.5
(1.1 - 3.0)
C max = peak concentration; AUC = area under curve; CL = clearance; Vss = volume of distribution at steady state; MRT = mean residence time
Effect of age and VWD type on the pharmacokinetics of Wilate:
Due to small sample size (in age or VWD type subsets) and high PK variability, it is difficult to conclude if age or type of VWD had any impact on the pharmacokinetics of Wilate.
Effect of gender on the pharmacokinetics of Wilate:
Based on PK data of Wilate from 8 males and 12 females, it appears that the females (4.35 ± 1.54 mL/hr/kg) had higher clearance of VWF:RCo than the males (3.16 ± 1.19 mL/hr/kg). The clinical significance of this finding is not known.
-
14 CLINICAL STUDIES
Clinical efficacy of Wilate in the control of bleeding in patients with VWD was determined in four prospective clinical studies. This included treatment of 1068 bleeding episodes (BEs). Data were obtained from 70 VWD patients, of which 37 were type 3. BEs are summarized in Table 5 . The treated BEs were analyzed for efficacy using a set of objective criteria in addition to a subjective 4-point hemostatic efficacy scale (excellent, good, moderate and none). In assessing the efficacy using these objective criteria, the treatment of a bleeding episode was classified as a success only if none of the criteria listed below was fulfilled:
- the episode was additionally treated with another VWF-containing product (excluding whole blood),
- the patient received a blood transfusion during the episode,
- follow-up treatment with a daily dosage of Wilate that was equal or more than 50% (≥ 50%) above the initial dose (for bleeding episodes with more than 1 day of treatment),
- treatment duration of more than 4 days (> 4 days) in cases of severe bleeding (other than gastrointestinal),
- treatment duration of more than 3 days (> 3 days) in cases of moderate bleeding (other than gastrointestinal),
- treatment duration of more than 2 days (> 2 days) in cases of minor bleeding (other than gastrointestinal),
- the last efficacy rating of the bleeding episode was 'moderate' or 'none'.
Among the 70 VWD patients administered Wilate in clinical studies (excluding the PK study), 45 of them received on demand treatment for BEs. Using the above objective criteria, corresponding efficacy for each bleeding event was rated as being successful in 84% of the episodes. In these 45 patients with BEs, 93% of the successfully treated BEs occurred in VWD type 3 patients (n=25).
Table 5 Proportion of successful treatments of bleeding episodes with Wilate (n=45)
95% CI Episodes*
Successful
% Successes
Lower CL
Upper CL
1068
898
84.1
81.8
86.2
The dosing information for the 972 successfully treated “bleeding episodes” (1423 infusions) for regional bleeding is summarized in Table 6 . For the purpose of assigning success/failure to regional bleeding that occurred at the same time, the bleeding at different sites over the same time span would be counted as separate BEs. Thus, the number of these “episodes” would be different from that in the overall evaluation for success/failure of Wilate in the treatment of bleeding episodes in Table 5 .
Table 6 Administered dosages (VWF:RCo in IU/kg) in bleeding episodes* successfully treated with Wilate: Mean ± SD (Range) (n=45)
Location All Doses considered Initial Dose Subsequent Doses # of infusions
Dose: Mean ± SD (Range)
# of infusions
Dose: Mean ± SD (Range)
# of infusions
Dose: Mean ± SD (Range)
Joints
801
26 ± 12
(7 - 69)
542
28 ± 13
(7 - 69)
259
21 ± 10
(7 - 60)
Epistaxis
132
24 ± 11
(8 - 78)
91
25 ± 10
(13 - 78)
41
22 ± 14
(8 - 77)
GI Tract
125
40 ± 20
(9 - 76)
64
43 ± 19
(9 - 76)
61
36 ± 21
(9 - 76)
Oral
41
26 ± 14
(8 - 80)
33
27 ± 14
(10 - 80)
8
24 ± 18
(8 - 60)
Gynecologic
87
27 ± 14
(9 - 77)
52
28 ± 17
(12 - 77)
35
26 ± 9
(9 - 52)
Other**
237
23 ± 12
(10 - 95)
189
24 ± 12
(12 - 95)
48
20 ± 13
(10 - 95)
**“Other” Includes mostly muscle bleeds, hematuria, ecchymosis, hematoma and other miscellaneous sites of bleeding
The majority of BEs were treated for 1-3 days. In patients with GI bleeds, the duration for product use to control bleeding could be much longer (up to 7 days).
For pediatric patients (≤16 yrs), a summary of the number of BEs treated and corresponding objective efficacy ratings are provided in Table 7 .
Table 7 Efficacy in bleeding episodes in pediatric population (5 to 16 yrs) (n=11) – Proportion of successful treatments of bleeding episodes with Wilate
95% CI Episodes*
Successful
% Successes
Lower CL
Upper CL
234
205
87.6
82.7
91.5
The dosing information for the 211 successfully treated bleeding episodes (289 infusions) is summarized in Table 8 . Multiple bleeding sites are counted as separate episodes.
Table 8 Administered dosages (VWF:RCo in IU/kg) in bleeding episodes* successfully treated with Wilate in pediatric population (5 to 16 yrs) (n=11): Mean ± SD (Range)
Location All Doses considered Initial Dose Subsequent Doses # of infusions
Dose: Mean ± SD (Range)
# of infusions
Dose: Mean ± SD (Range)
# of infusions
Dose: Mean ± SD (Range)
Joints
158
30 ± 13
(12 - 69)
117
32 ± 13
(14 - 69)
41
25 ± 9
(12 - 62)
Epistaxis
30
27 ± 14
(12 - 77)
25
25 ± 10
(14 - 52)
5
37 ± 25
(12 - 77)
GI Tract
1
22
(N/A)
1
22 (N/A)
0
N/A
Oral
23
25 ± 8
(16 - 52)
21
24 ± 8
(16 - 52)
2
25 ± 13
(16 - 35)
Gynecologic
58
27 ± 13
(12 - 69)
33
27 ± 16
(12 - 69)
25
26 ± 8
(12 - 52)
Other*
19
25 ± 7
(16 - 37)
14
27 ± 7
(19 - 37)
5
19 ± 4
(16 - 26)
**“Other” Includes mostly muscle bleeds, hematuria, ecchymosis, hematoma and other miscellaneous sites of bleeding
-
15 REFERENCES
- Mannucci P.M.: Venous thromboembolism in von Willebrand disease. Thromb Haemost 2002;88:378-379
- Mollison.P.L., Engelfriet C.P., Contreras M.: Some unfavourable effects of transfusion; in Klein H.G., Anstee D.J. (eds): Mollison's Blood Transfusion in Clinical Medicine. Blackwell Publishing, 2005, pp 666-700
- Mannucci P.M., Federici A.B.: Antibodies to von Willebrand factor in von Willebrand disease; in Aledort L.M. (ed): Inhibitors to Coagulation Factors. New York, Plenum Press, 1995, pp 87-92
- Y. Kasper, C.K., Kipnis, S.A. Hepatitis and Clotting Factor Concentrates. JAMA1972; 221:510
- X. Biggs, R. Jaundice and Antibodies Directed Against Factors VIII and IX in Patients Treated for Haemophilia or Christmas Disease in the United Kingdom. Br J Haematol 1974; 26:313-329
- Azzi A., Morfini M., Mannucci P.M.: The transfusion-associated transmission of parvovirus B19. Transfus.Med.Rev. 1999;13:194-204
- Stadler M., et al. Characterisation of a novel high-purity, double virus inactivated von Willebrand Factor and Factor VIII concentrate (Wilate). DOI: 10.1016/j.biologicals. Biologicals 2006, 34:281-288 1-8.
- Mannucci P.M.: Treatment of von Willebrand's disease. New England Journal of Medicine 2004;351:683-694
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC Number Size Protein Amount 67467-182-01
67467-182-02
500 IU VWF:RCo and 500 IU FVIII activities in 5 mL
1000 IU VWF:RCo and 1000 IU FVIII activities in 10 mL
≤ 7.5 mg
≤ 15.0 mg
- Wilate is supplied in a package with a single-dose vial of powder and a vial of diluent (Water for Injection with 0.1% Polysorbate 80), together with a Mix2VialTM transfer device, a 10-mL syringe, an infusion set and two alcohol swabs.
- Each vial of Wilate contains the labeled amount of IU of VWF:RCo activity as measured using a manual agglutination method, and IU of FVIII activity measured with a chromogenic substrate assay.
- Components used in the packaging of Wilate contain no latex.
- Store Wilate for up to 36 months at +2°C to +8°C (36°F to 46°F) protected from light from the date of manufacture. Within this period, Wilate may be stored for a period of up to 6 months at room temperature (maximum of +25°C or 77°F). The starting date of room temperature storage should be clearly recorded on the product carton. Once stored at room temperature, the product must not be returned to the refrigerator. The shelf-life then expires after the storage at room temperature, or the expiration date on the product vial, whichever is earliest. Do not freeze.
- Do not use after the expiration date.
- Store in the original container to protect from light.
- Reconstituted the Wilate powder only directly before injection. Use the solution immediately after reconstitution. Use the reconstituted solution on one occasion only, and discard any remaining solution.
-
17 PATIENT COUNSELING INFORMATION
- Inform patients of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If allergic symptoms occur, patients should discontinue the administration immediately and contact their physician [see Warnings and Precautions ( 5.1 )].
- Inform patients that undergoing multiple treatments with Wilate may increase the risk of thrombotic events thereby requiring frequent monitoring of plasma VWF:RCo and FVIII activities. [see Warnings and Precautions ( 5.2 )].
- Inform patients that there is a potential of developing inhibitors to VWF, leading to an inadequate clinical response. Thus, if the expected VWF activity plasma levels are not attained, or if bleeding is not controlled with an adequate dose or repeated dosing, contact the treating physician.[ 2 ] [see Warnings and Precautions ( 5.3 )].
- Inform patients that despite procedures for screening donors and plasma as well as those for inactivation or removal of infectious agents, the possibility of transmitting infective agents with plasma-derived products cannot be totally excluded [see Warnings and Precautions ( 5.4 )].
Manufactured by:
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
A-1100 Vienna, Austria
U.S. License No. 1646
Distributed by:
Octapharma USA Inc.
121 River Street, 12th floor
Hoboken, NJ 07030
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
von Willebrand Factor/Coagulation Factor VIII Complex (Human)
Octapharma Pharmazeutika Produktionsges.m.b.H
500IU/5mL
NDC: 67467-182-01

1000IU/10mL
NDC: 67467-182-02

-
INGREDIENTS AND APPEARANCE
WILATE - VON WILLEBRAND FACTOR/COAGULATION FACTOR VIII COMPLEX (HUMAN)
von willebrand factor/coagulation factor viii complex (human) powder, for solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 67467-182 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR HUMAN (UNII: 839MOZ74GK) (ANTIHEMOPHILIC FACTOR HUMAN - UNII:839MOZ74GK) ANTIHEMOPHILIC FACTOR HUMAN 100 [iU] in 1 mL VON WILLEBRAND FACTOR HUMAN (UNII: ZE22NE22F1) (VON WILLEBRAND FACTOR HUMAN - UNII:ZE22NE22F1) VON WILLEBRAND FACTOR HUMAN 100 [iU] in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67467-182-02 10 mL in 1 VIAL 2 NDC: 67467-182-01 5 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125251 12/04/2009 Labeler - Octapharma Pharmazeutika Produktionsgesellschaft m.b.H. (301119178)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.