GLOSTRIPS- fluorescein sodium strip
GloStrips by
Drug Labeling and Warnings
GloStrips by is a Prescription medication manufactured, distributed, or labeled by Nomax Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- INDICATIONS
-
DIRECTIONS FOR USE
To ensure full fluorescence and patient comfort, the GloStrip® impregnated tip should be moistened with one or two drops of sterile, isotonic saline or irrigating solution before application.
Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
INSTRUCTIONS FOR OPENING STERILE GLOSTRIPS®
1.Grasp free tab ends of wrapping and slowly pull apart. When the white paper handle becomes visible, remove the GloStrip® from the envelope. 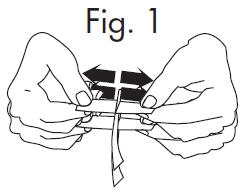
An Alternate Method of Opening
1.Grasp envelope firmly with two hands as shown in Fig. 2 below. Tear the envelope from both its edges to the strip handle. 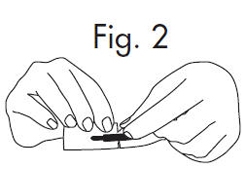
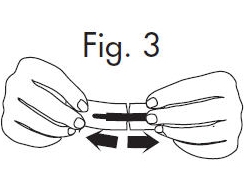
2. Hold the handle end of the GloStrip® with the left hand and the paper envelope without holding the tip with the right hand. See Fig. 3
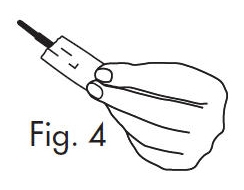
3. Separate the envelope at the tear, exposing the GloStrip® tip. See Fig. 4
-
PRINCIPAL DISPLAY PANEL - 300 Strip Carton
NDC: 51801-009-50
AMCON®
Laboratories, Inc.Fluorescein
GloStrips®1.01.0
1.0 mg Fluorescein Sodium
Ophthalmic Strips USP
300 Sterile Strips
-
INGREDIENTS AND APPEARANCE
GLOSTRIPS
fluorescein sodium stripProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51801-009 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluorescein Sodium (UNII: 93X55PE38X) (FLUORESCEIN - UNII:TPY09G7XIR) FLUORESCEIN 1 mg Product Characteristics Color ORANGE (paper is white and tip is orange) Score Shape RECTANGLE (with tapered end) Size 52mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51801-009-50 300 in 1 CARTON 07/30/2013 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC: 51801-009-40 100 in 1 CARTON 07/30/2013 2 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 07/30/2013 Labeler - Nomax Inc. (103220273) Establishment Name Address ID/FEI Business Operations Nomax Inc. 103220273 MANUFACTURE(51801-009)
Trademark Results [GloStrips]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GLOSTRIPS 78139112 2760506 Live/Registered |
Amcon Laboratories, Inc. 2002-06-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.