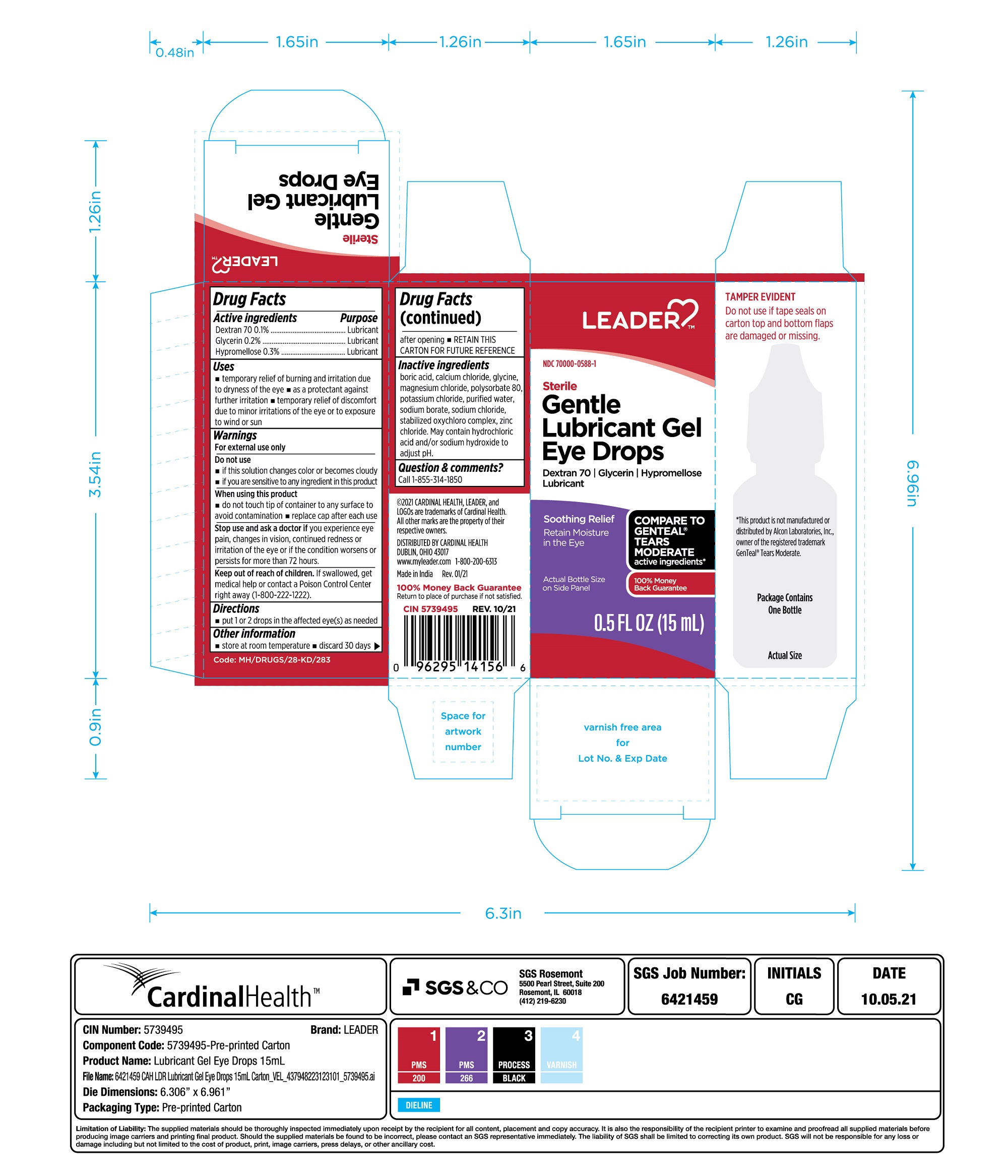
Gentle Lubricant eye drops
Sterile Gentle Tears by
Drug Labeling and Warnings
Sterile Gentle Tears by is a Otc medication manufactured, distributed, or labeled by CARDINAL HEALTH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
STERILE GENTLE TEARS LEADER- dextran 70, glycerin, hypromellose solution/ drops
CARDINAL HEALTH
----------
Gentle Lubricant eye drops
Uses
- temporary relief of burning and irritation due to dryness of the eye
- as a protectant against further irritation
- temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
Do not use
- if this solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if you experience any of the following:
- you feel eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
| STERILE GENTLE TEARS
LEADER
dextran 70, glycerin, hypromellose solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - CARDINAL HEALTH (063997360) |
Revised: 1/2024
Document Id: 0fa82684-0906-5189-e063-6394a90aa7fc
Set id: d08ab451-e8d5-28bb-e053-2995a90a4c03
Version: 5
Effective Time: 20240123
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.