SANDOSTATIN LAR DEPOT- octreotide acetate kit
Sandostatin LAR Depot by
Drug Labeling and Warnings
Sandostatin LAR Depot by is a Prescription medication manufactured, distributed, or labeled by Novartis Pharmaceuticals Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SANDOSTATIN LAR DEPOT safely and effectively. See full prescribing information for SANDOSTATIN LAR DEPOT.
SANDOSTATIN® LAR DEPOT (octreotide acetate) for injectable suspension, for gluteal intramuscular use
Initial U.S. Approval: 1988
RECENT MAJOR CHANGES
Warnings and Precautions (5.1)
4/2019
INDICATIONS AND USAGE
SANDOSTATIN LAR DEPOT is a somatostatin analogue indicated for:
Treatment in patients who have responded to and tolerated Sandostatin Injection subcutaneous injection for:
DOSAGE AND ADMINISTRATION
Patients Not Currently Receiving Sandostatin Injection Subcutaneously:
-
Acromegaly: 50 mcg three times daily Sandostatin Injection subcutaneously for 2 weeks followed by SANDOSTATIN LAR DEPOT 20 mg intragluteally every 4 weeks for 3 months (2.1)
- Carcinoid Tumors and VIPomas: Sandostatin Injection subcutaneously 100-600 mcg/day in 2-4 divided doses for 2 weeks followed by SANDOSTATIN LAR DEPOT 20 mg every 4 weeks for 2 months (2.2)
Patients Currently Receiving Sandostatin Injection Subcutaneously:
-
Acromegaly: 20 mg every 4 weeks for 3 months (2.1)
- Carcinoid Tumors and VIPomas: 20 mg every 4 weeks for 2 months (2.2)
Renal Impairment, Patients on Dialysis: 10 mg every 4 weeks (2.3)
Hepatic Impairment, Patients With Cirrhosis: 10 mg every 4 weeks (2.4)
DOSAGE FORMS AND STRENGTHS
For injectable suspension: strengths 10 mg per 6 mL, 20 mg per 6 mL, or 30 mg per 6 mL vials (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
-
Cholelithiasis and Complications of Cholelithiasis: Monitor periodically. Discontinue if complications of cholelithiasis are suspected (5.1)
-
Glucose Metabolism: Hypoglycemia or hyperglycemia may occur. Glucose monitoring is recommended and antidiabetic treatment may need adjustment. (5.2)
-
Thyroid Function: Hypothyroidism may occur. Monitor thyroid levels periodically. (5.3)
- Cardiac Function: Bradycardia, arrhythmia, or conduction abnormalities may occur. Use with caution in at-risk patients. (5.4)
ADVERSE REACTIONS
The most common adverse reactions, occurring in ≥ 20% of patients are:
-
Acromegaly: diarrhea, cholelithiasis, abdominal pain, flatulence (6.1)
- Carcinoid Syndrome: back pain, fatigue, headache, abdominal pain, nausea, dizziness (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
The following drugs require monitoring and possible dose adjustment when used with SANDOSTATIN LAR DEPOT: cyclosporine, insulin, oral hypoglycemic agents, beta-blockers, and bromocriptine. (7)
USE IN SPECIFIC POPULATIONS
Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy. (8.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2019
-
Acromegaly: 50 mcg three times daily Sandostatin Injection subcutaneously for 2 weeks followed by SANDOSTATIN LAR DEPOT 20 mg intragluteally every 4 weeks for 3 months (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Acromegaly
1.2 Carcinoid Tumors
1.3 Vasoactive Intestinal Peptide Tumors (VIPomas)
1.4 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Acromegaly
2.2 Carcinoid Tumors and VIPomas
2.3 Special Populations: Renal Impairment
2.4 Special Populations: Hepatic Impairment – Cirrhotic Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cholelithiasis and Complications of Cholelithiasis
5.2 Hyperglycemia and Hypoglycemia
5.3 Thyroid Function Abnormalities
5.4 Cardiac Function Abnormalities
5.5 Nutrition
5.6 Monitoring: Laboratory Tests
5.7 Drug Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cyclosporine
7.2 Insulin and Oral Hypoglycemic Drugs
7.3 Bromocriptine
7.4 Other Concomitant Drug Therapy
7.5 Drug Metabolism Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment-Cirrhotic Patients
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Reproductive Toxicology Studies
14 CLINICAL STUDIES
14.1 Acromegaly
14.2 Carcinoid Syndrome
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
SANDOSTATIN LAR DEPOT 10 mg, 20 mg, and 30 mg is indicated in patients in whom initial treatment with Sandostatin Injection has been shown to be effective and tolerated.
1.1 Acromegaly
Long-term maintenance therapy in acromegalic patients who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy, is not an option. The goal of treatment in acromegaly is to reduce GH and IGF-1 levels to normal [see Clinical Studies (14), Dosage and Administration (2)].
1.2 Carcinoid Tumors
Long-term treatment of the severe diarrhea and flushing episodes associated with metastatic carcinoid tumors.
-
2 DOSAGE AND ADMINISTRATION
- SANDOSTATIN LAR DEPOT should be administered by a trained healthcare provider. It is important to closely follow the mixing instructions included in the packaging. SANDOSTATIN LAR DEPOT must be administered immediately after mixing.
-
Do not directly inject diluent without preparing suspension.
- The recommended needle size for administration of SANDOSTATIN LAR DEPOT is the 1½” 19 gauge safety injection needle (supplied in the drug product kit). For patients with a greater skin to muscle depth, a size 2” 19 gauge needle (not supplied) may be used.
- SANDOSTATIN LAR DEPOT should be administered intramuscularly (IM) in the gluteal region at 4-week intervals. Administration of SANDOSTATIN LAR DEPOT at intervals greater than 4 weeks is not recommended.
- Injection sites should be rotated in a systematic manner to avoid irritation. Deltoid injections should be avoided due to significant discomfort at the injection site when given in that area.
- SANDOSTATIN LAR DEPOT should never be administered intravenously or subcutaneously.
The following dosage regimens are recommended.
2.1 Acromegaly
Patients Not Currently Receiving Octreotide Acetate
Patients not currently receiving octreotide acetate should begin therapy with Sandostatin Injection given subcutaneously in an initial dose of 50 mcg three times daily which may be titrated. Most patients require doses of 100 mcg to 200 mcg three times daily for maximum effect but some patients require up to 500 mcg three times daily.
Patients should be maintained on Sandostatin Injection subcutaneous for at least 2 weeks to determine tolerance to octreotide. Patients who are considered to be “responders” to the drug, based on GH and IGF-1 levels and who tolerate the drug, can then be switched to SANDOSTATIN LAR DEPOT in the dosage scheme described below (Patients Currently Receiving Sandostatin Injection).
Patients Currently Receiving Sandostatin Injection
Patients currently receiving Sandostatin Injection can be switched directly to SANDOSTATIN LAR DEPOT in a dose of 20 mg given IM intragluteally at 4-week intervals for 3 months. After 3 months, dosage may be adjusted as follows:
- GH ≤ 2.5 ng/mL, IGF-1 normal, and clinical symptoms controlled: maintain SANDOSTATIN LAR DEPOT dosage at 20 mg every 4 weeks
- GH > 2.5 ng/mL, IGF-1 elevated, and/or clinical symptoms uncontrolled, increase SANDOSTATIN LAR DEPOT dosage to 30 mg every 4 weeks
- GH ≤ 1 ng/mL, IGF-1 normal, and clinical symptoms controlled, reduce SANDOSTATIN LAR DEPOT dosage to 10 mg every 4 weeks
- If GH, IGF-1, or symptoms are not adequately controlled at a dose of 30 mg, the dose may be increased to 40 mg every 4 weeks. Doses higher than 40 mg are not recommended.
In patients who have received pituitary irradiation, SANDOSTATIN LAR DEPOT should be withdrawn yearly for approximately 8 weeks to assess disease activity. If GH or IGF-1 levels increase and signs and symptoms recur, SANDOSTATIN LAR DEPOT therapy may be resumed.
2.2 Carcinoid Tumors and VIPomas
Patients not Currently Receiving Octreotide Acetate
Patients not currently receiving octreotide acetate should begin therapy with Sandostatin Injection given subcutaneously. The suggested daily dosage for carcinoid tumors during the first 2 weeks of therapy ranges from 100-600 mcg/day in 2-4 divided doses (mean daily dosage is 300 mcg). Some patients may require doses up to 1500 mcg/day. The suggested daily dosage for VIPomas is 200-300 mcg in 2-4 divided doses (range 150-750 mcg); dosage may be adjusted on an individual basis to control symptoms but usually doses above 450 mcg/day are not required.
Sandostatin Injection should be continued for at least 2 weeks. Thereafter, patients who are considered “responders” to octreotide acetate, and who tolerate the drug, may be switched to SANDOSTATIN LAR DEPOT in the dosage regimen as described below (Patients Currently Receiving Sandostatin Injection).
Patients Currently Receiving Sandostatin Injection
Patients currently receiving Sandostatin Injection can be switched to SANDOSTATIN LAR DEPOT in a dosage of 20 mg given IM intragluteally at 4-week intervals for 2 months. Because of the need for serum octreotide to reach therapeutically effective levels following initial injection of SANDOSTATIN LAR DEPOT, carcinoid tumor and VIPoma patients should continue to receive Sandostatin Injection subcutaneously for at least 2 weeks in the same dosage they were taking before the switch. Failure to continue subcutaneous injections for this period may result in exacerbation of symptoms (some patients may require 3 or 4 weeks of such therapy).
After 2 months, dosage may be adjusted as follows:
If symptoms are adequately controlled, consider a dose reduction to 10 mg for a trial period. If symptoms recur, dosage should then be increased to 20 mg every 4 weeks. Many patients can, however, be satisfactorily maintained at a 10-mg dose every 4 weeks.
- If symptoms are not adequately controlled, increase SANDOSTATIN LAR DEPOT to 30 mg every 4 weeks. Patients who achieve good control on a 20-mg dose may have their dose lowered to 10 mg for a trial period. If symptoms recur, dosage should then be increased to 20 mg every 4 weeks.
- Dosages higher than 30 mg are not recommended.
Despite good overall control of symptoms, patients with carcinoid tumors and VIPomas often experience periodic exacerbation of symptoms (regardless of whether they are being maintained on Sandostatin Injection or SANDOSTATIN LAR DEPOT). During these periods, they may be given Sandostatin Injection subcutaneously for a few days at the dosage they were receiving prior to switching to SANDOSTATIN LAR DEPOT. When symptoms are again controlled, the Sandostatin Injection subcutaneous can be discontinued.
2.3 Special Populations: Renal Impairment
In patients with renal failure requiring dialysis, the starting dose should be 10 mg every 4 weeks. In other patients with renal impairment, the starting dose should be similar to a nonrenal patient (i.e., 20 mg every 4 weeks) [see Clinical Pharmacology (12)].
- SANDOSTATIN LAR DEPOT should be administered by a trained healthcare provider. It is important to closely follow the mixing instructions included in the packaging. SANDOSTATIN LAR DEPOT must be administered immediately after mixing.
-
3 DOSAGE FORMS AND STRENGTHS
SANDOSTATIN LAR DEPOT is available in single-use kits for injectable suspension containing a 6-mL vial of 10 mg, 20 mg, or 30 mg strength, a syringe containing 2 mL of diluent, one vial adapter, and one sterile 1½” 19 gauge safety injection needle. An instruction booklet for the preparation of drug suspension for injection is also included with each kit.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cholelithiasis and Complications of Cholelithiasis
SANDOSTATIN LAR DEPOT may inhibit gallbladder contractility and decrease bile secretion, which may lead to gallbladder abnormalities or sludge. There have been postmarketing reports of cholelithiasis (gallstones) resulting in complications, including cholecystitis, cholangitis, pancreatitis and requiring cholecystectomy in patients taking SANDOSTATIN LAR DEPOT [see Adverse Reactions (6)]. Patients should be monitored periodically. If complications of cholelithiasis are suspected, discontinue SANDOSTATIN LAR DEPOT and treat appropriately.
5.2 Hyperglycemia and Hypoglycemia
Octreotide alters the balance between the counter-regulatory hormones, insulin, glucagon, and growth hormone (GH), which may result in hypoglycemia or hyperglycemia. Blood glucose levels should be monitored when SANDOSTATIN LAR DEPOT treatment is initiated, or when the dose is altered. Anti-diabetic treatment should be adjusted accordingly [see Adverse Reactions (6)].
5.3 Thyroid Function Abnormalities
Octreotide suppresses the secretion of thyroid-stimulating hormone (TSH), which may result in hypothyroidism. Baseline and periodic assessment of thyroid function (TSH, total and/or free T4) is recommended during chronic octreotide therapy [see Adverse Reactions (6)].
5.4 Cardiac Function Abnormalities
In both acromegalic and carcinoid syndrome patients, bradycardia, arrhythmias and conduction abnormalities have been reported during octreotide therapy. Other ECG changes were observed such as QT prolongation, axis shifts, early repolarization, low voltage, R/S transition, early R wave progression, and nonspecific ST-T wave changes. The relationship of these events to octreotide acetate is not established because many of these patients have underlying cardiac disease. Dose adjustments in drugs such as beta-blockers that have bradycardic effects may be necessary. In one acromegalic patient with severe congestive heart failure (CHF), initiation of Sandostatin Injection-therapy resulted in worsening of CHF with improvement when drug was discontinued. Confirmation of a drug effect was obtained with a positive rechallenge [see Adverse Reactions (6)].
5.5 Nutrition
Octreotide may alter absorption of dietary fats.
Depressed vitamin B12 levels and abnormal Schilling tests have been observed in some patients receiving octreotide therapy, and monitoring of vitamin B12 levels is recommended during therapy with SANDOSTATIN LAR DEPOT.
Octreotide has been investigated for the reduction of excessive fluid loss from the GI tract in patients with conditions producing such a loss. If such patients are receiving total parenteral nutrition (TPN), serum zinc may rise excessively when the fluid loss is reversed. Patients on TPN and octreotide should have periodic monitoring of zinc levels.
5.6 Monitoring: Laboratory Tests
Laboratory tests that may be helpful as biochemical markers in determining and following patient response depend on the specific tumor. Based on diagnosis, measurement of the following substances may be useful in monitoring the progress of therapy [see Dosage and Administration (2.1, 2.2)].
Acromegaly: Growth Hormone, IGF-1 (somatomedin C)
Carcinoid: 5-HIAA (urinary 5-hydroxyindole acetic acid), plasma serotonin, plasma Substance P
VIPoma: VIP (plasma vasoactive intestinal peptide) baseline and periodic total and/or free T4 measurements should be performed during chronic therapy
5.7 Drug Interactions
Octreotide has been associated with alterations in nutrient absorption, so it may have an effect on absorption of orally administered drugs. Concomitant administration of octreotide injection with cyclosporine may decrease blood levels of cyclosporine [see Drug Interactions (7.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.
Acromegaly
The safety of SANDOSTATIN LAR DEPOT in the treatment of acromegaly has been evaluated in three Phase 3 studies in 261 patients, including 209 exposed for 48 weeks and 96 exposed for greater than 108 weeks. SANDOSTATIN LAR DEPOT was studied primarily in a double-blind, cross-over manner. Patients on subcutaneous Sandostatin Injection were switched to the LAR formulation followed by an open-label extension. The population age range was 14-81 years old and 53% were female. Approximately 35% of these acromegaly patients had not been treated with surgery and/or radiation. Most patients received a starting dose of 20 mg every 4 weeks intramuscularly. Dose was up or down titrated based on efficacy and tolerability to a final dose between 10-60 mg every 4 weeks. Table 1 below reflects adverse events from these studies regardless of presumed causality to study drug.
Table 1. Adverse Events Occurring in ≥ 10% of Acromegalic Patients in the Phase 3 Studies AEs, adverse events. WHO Preferred Term Phase 3 Studies (Pooled)
Number (%) of Subjects with AEs
10 mg/20 mg/30 mg
(n = 261)
n (%)Diarrhea 93 (35.6) Abdominal Pain 75 (28.7) Flatulence 66 (25.3) Influenza-Like Symptoms 52 (19.9) Constipation 46 (17.6) Headache 40 (15.3) Anemia 40 (15.3) Injection-Site Pain 36 (13.8) Cholelithiasis 35 (13.4) Hypertension 33 (12.6) Dizziness 30 (11.5) Fatigue 29 (11.1) The safety of SANDOSTATIN LAR DEPOT in the treatment of acromegaly was also evaluated in a postmarketing randomized Phase 4 study. One-hundred four (104) patients were randomized to either pituitary surgery or 20 mg of SANDOSTATIN LAR DEPOT. All the patients were treatment naïve (‘de novo’). Crossover was allowed according to treatment response and a total of 76 patients were exposed to Sandostatin LAR. Approximately half of the patients initially randomized to SANDOSTATIN LAR DEPOT were exposed to SANDOSTATIN LAR DEPOT up to 1 year. The population age range was between 20-76 years old, 45% were female, 93% were Caucasian, and 1% Black. The majority of these patients were exposed to 30 mg every 4 weeks. Table 2 below reflects the adverse events occurring in this study regardless of presumed causality to study drug.
Table 2. Adverse Events Occurring in ≥ 10% of Acromegalic Patients in Phase 4 Study WHO Preferred Term Phase 4 Study
SANDOSTATIN LAR DEPOT
N = 76
n (%)Phase 4 Study
Surgery
N = 64
n (%)Diarrhea 36 (47.4) 2 (3.1) Cholelithiasis 29 (38.2) 3 (4.7) Abdominal Pain 19 (25.0) 2 (3.1) Nausea 12 (15.8) 5 (7.8) Alopecia 10 (13.2) 5 (7.8) Injection-Site Pain 9 (11.8) 0 Abdominal Pain Upper 8 (10.5) 0 Headache 8 (10.5) 6 (9.4) Epistaxis 0 7 (10.9) Gallbladder Abnormalities
Single doses of Sandostatin Injection have been shown to inhibit gallbladder contractility and decrease bile secretion in normal volunteers. In clinical trials with Sandostatin Injection (primarily patients with acromegaly or psoriasis) in patients who had not previously received octreotide, the incidence of biliary tract abnormalities was 63% (27% gallstones, 24% sludge without stones, 12% biliary duct dilatation). The incidence of stones or sludge in patients who received Sandostatin Injection for 12 months or longer was 52%. The incidence of gallbladder abnormalities did not appear to be related to age, sex, or dose but was related to duration of exposure.
In clinical trials, 52% of acromegalic patients, most of whom received SANDOSTATIN LAR DEPOT for 12 months or longer, developed new biliary abnormalities including gallstones, microlithiasis, sediment, sludge, and dilatation. The incidence of new cholelithiasis was 22%, of which 7% were microstones.
Across all trials, a few patients developed acute cholecystitis, ascending cholangitis, biliary obstruction, cholestatic hepatitis, or pancreatitis during octreotide therapy or following its withdrawal. One patient developed ascending cholangitis during Sandostatin Injection therapy and died. Despite the high incidence of new gallstones in patients receiving octreotide, 1% of patients developed acute symptoms requiring cholecystectomy.
Glucose Metabolism - Hypoglycemia/Hyperglycemia
In acromegaly patients treated with either Sandostatin Injection or SANDOSTATIN LAR DEPOT, hypoglycemia occurred in approximately 2% and hyperglycemia in approximately 15% of patients [see Warnings and Precautions (5.2)].
Hypothyroidism
In acromegaly patients receiving Sandostatin Injection, 12% developed biochemical hypothyroidism, 8% developed goiter, and 4% required initiation of thyroid replacement therapy while receiving Sandostatin Injection. In acromegalic patients treated with SANDOSTATIN LAR DEPOT, hypothyroidism was reported as an adverse event in 2% and goiter in 2%. Two patients receiving SANDOSTATIN LAR DEPOT required initiation of thyroid hormone replacement therapy [see Warnings and Precautions (5.3)].
Cardiac
In acromegalic patients, sinus bradycardia (< 50 bpm) developed in 25%; conduction abnormalities occurred in 10% and arrhythmias developed in 9% of patients during Sandostatin Injection-therapy. The relationship of these events to octreotide acetate is not established because many of these patients have underlying cardiac disease [see Warnings and Precautions (5.4)].
Gastrointestinal
The most common symptoms are gastrointestinal. The overall incidence of the most frequent of these symptoms in clinical trials of acromegalic patients treated for approximately 1 to 4 years is shown in Table 3.
Table 3. Number (%) of Acromegalic Patients With Common GI Adverse Events Adverse Event Sandostatin Injection S.C.
Three Times Daily
n = 114SANDOSTATIN LAR DEPOT
Every 28 Days
n = 261n % n % Diarrhea 66 (57.9) 95 (36.4) Abdominal Pain or Discomfort 50 (43.9) 76 (29.1) Nausea 34 (29.8) 27 (10.3) Flatulence 15 (13.2) 67 (25.7) Constipation 10 (8.8) 49 (18.8) Vomiting 5 (4.4) 17 (6.5) Only 2.6% of the patients on Sandostatin Injection in US clinical trials discontinued therapy due to these symptoms. No acromegalic patient receiving SANDOSTATIN LAR DEPOT discontinued therapy for a GI event.
In patients receiving SANDOSTATIN LAR DEPOT, the incidence of diarrhea was dose related. Diarrhea, abdominal pain, and nausea developed primarily during the first month of treatment with SANDOSTATIN LAR DEPOT. Thereafter, new cases of these events were uncommon. The vast majority of these events were mild-to-moderate in severity.
In rare instances, gastrointestinal adverse effects may resemble acute intestinal obstruction, with progressive abdominal distention, severe epigastric pain, abdominal tenderness, and guarding.
Dyspepsia, steatorrhea, discoloration of feces, and tenesmus were reported in 4%-6% of patients.
In a clinical trial of carcinoid syndrome, nausea, abdominal pain, and flatulence were reported in 27%-38% and constipation or vomiting in 15%-21% of patients treated with SANDOSTATIN LAR DEPOT. Diarrhea was reported as an adverse event in 14% of patients, but since most of the patients had diarrhea as a symptom of carcinoid syndrome, it is difficult to assess the actual incidence of drug-related diarrhea.
Pain at the Injection Site
Pain on injection, which is generally mild-to-moderate, and short-lived (usually about 1 hour) is dose related, being reported by 2%, 9%, and 11% of acromegalic patients receiving doses of 10 mg, 20 mg, and 30 mg, respectively, of SANDOSTATIN LAR DEPOT. In carcinoid patients, where a diary was kept, pain at the injection site was reported by about 20%-25% at a 10-mg dose and about 30%-50% at the 20-mg and 30-mg dose.
Antibodies to Octreotide
Studies to date have shown that antibodies to octreotide develop in up to 25% of patients treated with octreotide acetate. These antibodies do not influence the degree of efficacy response to octreotide; however, in two acromegalic patients who received Sandostatin Injection, the duration of GH suppression following each injection was about twice as long as in patients without antibodies. It has not been determined whether octreotide antibodies will also prolong the duration of GH suppression in patients being treated with SANDOSTATIN LAR DEPOT.
Carcinoid and VIPomas
The safety of SANDOSTATIN LAR DEPOT in the treatment of carcinoid tumors and VIPomas has been evaluated in one Phase 3 study. Study 1 randomized 93 patients with carcinoid syndrome to SANDOSTATIN LAR DEPOT 10 mg, 20 mg, or 30 mg in a blind fashion or to open-label Sandostatin Injection subcutaneously. The population age range was between 25-78 years old and 44% were female, 95% were Caucasian and 3% Black. All the patients had symptom control on their previous Sandostatin subcutaneous treatment. 80 patients finished the initial 24 weeks of Sandostatin exposure in Study 1. In Study 1, comparable numbers of patients were randomized to each dose. Table 4 below reflects the adverse events occurring in ≥ 15% of patients regardless of presumed causality to study drug.
Table 4. Adverse Events Occurring in ≥ 15% of Carcinoid Tumor and VIPoma Patients in Study 1 Number (%) of Subjects With AEs
(n = 93)WHO Preferred Term S.C.
N = 2610 mg
N = 2220 mg
N = 2030 mg
N = 25Abdominal Pain 8 (30.8) 8 (35.4) 2 (10.0) 5 (20.0) Arthropathy 5 (19.2) 2 (9.1) 3 (15.0) 2 (8.0) Back Pain 7 (26.9) 6 (27.3) 2 (10.0) 2 (8.0) Dizziness 4 (15.4) 4 (18.2) 4 (20.0) 5 (20.0) Fatigue 3 (11.5) 7 (31.8) 2 (10.0) 2 (8.0) Flatulence 3 (11.5) 2 (9.1) 2 (10.0) 4 (16.0) Generalized Pain 4 (15.4) 2 (9.1) 3 (15.0) 1 (4.0) Headache 5 (19.2) 4 (18.2) 6 (30.0) 4(16.0) Musculoskeletal Pain 4 (15.4) 0 1 (5.0) 0 Myalgia 0 4 (18.2) 1 (5.0) 1 (4.0) Nausea 8 (30.8) 9 (40.9) 6 (30.0) 6 (24.0) Pruritus 0 4 (18.2) 0 0 Rash 1 (3.8) 0 3 (15.0) 0 Sinusitis 4 (15.4) 0 1 (5.0) 3 (12.0) URTI 6 (23.1) 4 (18.2) 2 (10.0) 3 (12.0) Vomiting 3 (11.5) 0 0 4 (16.0) Gallbladder Abnormalities
In clinical trials, 62% of malignant carcinoid patients, who received SANDOSTATIN LAR DEPOT for up to 18 months, developed new biliary abnormalities including jaundice, gallstones, sludge, and dilatation. New gallstones occurred in a total of 24% of patients.
Glucose Metabolism - Hypoglycemia/Hyperglycemia
In carcinoid patients, hypoglycemia occurred in 4% and hyperglycemia in 27% of patients treated with SANDOSTATIN LAR DEPOT [see Warnings and Precautions (5.2)].
Hypothyroidism
In carcinoid patients, hypothyroidism has only been reported in isolated patients and goiter has not been reported [see Warnings and Precautions (5.3)].
Cardiac
Electrocardiograms were performed only in carcinoid patients receiving SANDOSTATIN LAR DEPOT. In carcinoid syndrome patients, sinus bradycardia developed in 19%, conduction abnormalities occurred in 9%, and arrhythmias developed in 3%. The relationship of these events to octreotide acetate is not established because many of these patients have underlying cardiac disease [see Warnings and Precautions (5.4)].
Other Clinical Studies Adverse Events
Other clinically significant adverse events (relationship to drug not established) in acromegalic and/or carcinoid syndrome patients receiving SANDOSTATIN LAR DEPOT were malignant hyperpyrexia, cerebral vascular disorder, rectal bleeding, ascites, pulmonary embolism, pneumonia, and pleural effusion.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the postapproval use of SANDOSTATIN LAR DEPOT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic: pancytopenia, thrombocytopenia
Cardiac: myocardial infarction, cardiac arrest, atrial fibrillation
Ear and labryinth: deafness
Endocrine: diabetes insipidus, adrenal insufficiency in patients 18 months of age and under, pituitary apoplexy
Eye: glaucoma, visual field defect, scotoma, retinal vein thrombosis
Gastrointestinal: intestinal obstruction, peptic/gastric ulcer, abdomen enlarged
General and administration site: generalized edema, facial edema
Hepatobiliary: gallbladder polyp, fatty liver, hepatitis
Immune: anaphylactoid reactions including anaphylactic shock
Infections and infestations: appendicitis
Laboratory abnormalities: increased liver enzymes, CK increased, creatinine increased
Metabolism and nutrition: diabetes mellitus
Musculoskeletal: arthritis, joint effusion, Raynaud’s syndrome
Nervous System: convulsions, aneurysm, intracranial hemorrhage, hemiparesis, paresis, suicide attempt, paranoia, migraines, Bell’s palsy, aphasia
Renal and urinary: renal failure, renal insufficiency
Reproductive and breast: gynecomastia, galactorrhea, libido decrease, breast carcinoma
Respiratory: status asthmaticus, pulmonary hypertension, pulmonary nodule, pneumothorax aggravated
Skin and subcutaneous tissue: urticaria, cellulitis, petechiae
Vascular: orthostatic hypotension, hematuria, gastrointestinal hemorrhage, arterial thrombosis of the arm
-
7 DRUG INTERACTIONS
7.1 Cyclosporine
Concomitant administration of octreotide injection with cyclosporine may decrease blood levels of cyclosporine and result in transplant rejection.
7.2 Insulin and Oral Hypoglycemic Drugs
Octreotide inhibits the secretion of insulin and glucagon. Therefore, blood glucose levels should be monitored when SANDOSTATIN LAR DEPOT treatment is initiated or when the dose is altered and anti-diabetic treatment should be adjusted accordingly.
7.3 Bromocriptine
Concomitant administration of octreotide and bromocriptine increases the availability of bromocriptine.
7.4 Other Concomitant Drug Therapy
Concomitant administration of bradycardia-inducing drugs (e.g., beta-blockers) may have an additive effect on the reduction of heart rate associated with octreotide. Dose adjustments of concomitant medication may be necessary.
Octreotide has been associated with alterations in nutrient absorption, so it may have an effect on absorption of orally administered drugs.
7.5 Drug Metabolism Interactions
Limited published data indicate that somatostatin analogs may decrease the metabolic clearance of compounds known to be metabolized by cytochrome P450 enzymes, which may be due to the suppression of growth hormone. Since it cannot be excluded that octreotide may have this effect, other drugs mainly metabolized by CYP3A4 and which have a low therapeutic index (e.g., quinidine, terfenadine) should therefore be used with caution.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited data with SANDOSTATIN LAR DEPOT in pregnant women are insufficient to inform a drug-associated risk for major birth defects and miscarriage. In animal reproduction studies, no-adverse-developmental-effects were observed with intravenous administration of octeotride to pregnant rats and rabbits during organogenesis at doses 7 and 13-times, respectively the maximum recommended human dose (MRHD) of 1500 mcg/day based on body surface area. Transient growth retardation, with no impact on postnatal development, was observed in rat offspring from a pre- and post-natal study of octreotide at intravenous doses below the MRHD based on body surface area (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
In embryo-fetal development studies in rats and rabbits, pregnant animals received intravenous doses of octreotide up to 1 mg/kg/day during the period of organogenesis. A slight reduction in body weight gain was noted in pregnant rats at 0.1 and 1 mg/kg/day. There were no maternal effects in rabbits or embryo-fetal effects in either species up to the maximum dose tested. At 1 mg/kg/day in rats and rabbits, the dose multiple was approximately 7 and 13 times, respectively, at the highest recommended human dose of 1500 mcg/day based on body surface area.
In a pre- and post-natal development rat study at intravenous doses of 0.02–1 mg/kg/day, a transient growth retardation of the offspring was observed at all doses which was possibly a consequence of growth hormone inhibition by octreotide. The doses attributed to the delayed growth are below the human dose of 1500 mcg/day, based on body surface area.
8.2 Lactation
Risk Summary
There is no information available on the presence of SANDOSTATIN LAR DEPOT in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. Studies show that octreotide administered subcutaneously passes into the milk of lactating rats; however, due to species-specific differences in lactation physiology, animal data may not reliably predict drug levels in human milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SANDOSTATIN LAR DEPOT, and any potential adverse effects on the breastfed child from SANDOSTATIN LAR DEPOT or from the underlying maternal condition.
Data
Following a subcutaneous dose (1 mg/kg) of octreotide to lactating rats, transfer of octreotide into milk was observed at a low concentration compared to plasma (milk/plasma ratio of 0.009).
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as the therapeutic benefits of a reduction in GH levels and normalization of insulin-like growth factor 1 (IGF-1) concentration in acromegalic females treated with octeotride may lead to improved fertility.
8.4 Pediatric Use
Safety and efficacy of SANDOSTATIN LAR DEPOT in the pediatric population have not been demonstrated.
No formal controlled clinical trials have been performed to evaluate the safety and effectiveness of SANDOSTATIN LAR DEPOT in pediatric patients under 6 years of age. In postmarketing reports, serious adverse events, including hypoxia, necrotizing enterocolitis, and death, have been reported with Sandostatin use in children, most notably in children under 2 years of age. The relationship of these events to octreotide has not been established as the majority of these pediatric patients had serious underlying comorbid conditions.
The efficacy and safety of SANDOSTATIN LAR DEPOT was examined in a single randomized, double-blind, placebo-controlled, 6-month pharmacokinetics study in 60 pediatric patients age 6-17 years with hypothalamic obesity resulting from cranial insult. The mean octreotide concentration after 6 doses of 40 mg SANDOSTATIN LAR DEPOT administered by IM injection every four weeks was approximately 3 ng/mL. Steady-state concentrations were achieved after 3 injections of a 40 mg dose. Mean BMI increased 0.1 kg/m2 in SANDOSTATIN LAR DEPOT-treated subjects compared to 0.0 kg/m2 in saline control-treated subjects. Efficacy was not demonstrated. Diarrhea occurred in 11 of 30 (37%) patients treated with SANDOSTATIN LAR DEPOT. No unexpected adverse events were observed. However, with SANDOSTATIN LAR DEPOT 40 mg once a month, the incidence of new cholelithiasis in this pediatric population (33%) was higher than that seen in other adult indications such as acromegaly (22%) or malignant carcinoid syndrome (24%), where SANDOSTATIN LAR DEPOT was dosed at 10 to 30 mg once a month.
8.5 Geriatric Use
Clinical studies of Sandostatin did not include sufficient numbers of subjects age 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
In patients with renal failure requiring dialysis, the starting dose should be 10 mg. This dose should be up titrated based on clinical response and speed of response as deemed necessary by the physician. In patients with mild, moderate, or severe renal impairment there is no need to adjust the starting dose of Sandostatin. The maintenance dose should be adjusted thereafter based on clinical response and tolerability as in nonrenal patients [see Clinical Pharmacology (12)].
8.7 Hepatic Impairment-Cirrhotic Patients
In patients with established liver cirrhosis, the starting dose should be 10 mg. This dose should be up titrated based on clinical response and speed of response as deemed necessary by the physician. Once at a higher dose, patient should be maintained or dose adjusted based on response and tolerability as in any noncirrhotic patients [see Clinical Pharmacology (12)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Octreotide is the acetate salt of a cyclic octapeptide. It is a long-acting octapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin. Octreotide is known chemically as L-Cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1- (hydroxy-methyl) propyl]-, cyclic (2→7)-disulfide; [R-(R*,R*)].
The molecular weight of octreotide is 1019.3 g/mol (free peptide, C49H66N10O10S2) and its amino acid sequence is:
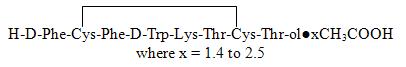
SANDOSTATIN LAR DEPOT is available in a vial containing the sterile drug product, which when mixed with diluent, becomes a suspension that is given as a monthly intragluteal injection. The octreotide is uniformly distributed within the microspheres which are made of a biodegradable glucose star polymer, D,L-lactic and glycolic acids copolymer. Sterile mannitol is added to the microspheres to improve suspendability.
SANDOSTATIN LAR DEPOT is available as: sterile 6-mL vials in 3 strengths delivering 10 mg, 20 mg, or 30 mg octreotide-free peptide. Each vial of SANDOSTATIN LAR DEPOT delivers:
*Equivalent to 10/20/30 mg octreotide base. Name of Ingredient 10 mg 20 mg 30 mg octreotide acetate 11.2 mg* 22.4 mg* 33.6 mg* D,L-lactic and glycolic acids copolymer 188.8 mg 377.6 mg 566.4 mg mannitol 41.0 mg 81.9 mg 122.9 mg Each syringe of diluent contains: carboxymethylcellulose sodium 14.0 mg mannitol 12.0 mg poloxamer 188 4.0 mg water for injection 2.0 mL -
12 CLINICAL PHARMACOLOGY
SANDOSTATIN LAR DEPOT is a long-acting dosage form consisting of microspheres of the biodegradable glucose star polymer, D,L-lactic and glycolic acids copolymer, containing octreotide. It maintains all of the clinical and pharmacological characteristics of the immediate-release dosage form Sandostatin Injection with the added feature of slow release of octreotide from the site of injection, reducing the need for frequent administration. This slow release occurs as the polymer biodegrades, primarily through hydrolysis. SANDOSTATIN LAR DEPOT is designed to be injected intramuscularly (intragluteally) once every 4 weeks.
12.1 Mechanism of Action
Octreotide exerts pharmacologic actions similar to the natural hormone, somatostatin. It is an even more potent inhibitor of growth hormone, glucagon, and insulin than somatostatin. Like somatostatin, it also suppresses LH response to GnRH, decreases splanchnic blood flow, and inhibits release of serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide.
By virtue of these pharmacological actions, octreotide has been used to treat the symptoms associated with metastatic carcinoid tumors (flushing and diarrhea), and VIP secreting adenomas (watery diarrhea).
12.2 Pharmacodynamics
Octreotide substantially reduces and in many cases can normalize growth hormone and/or IGF-1 (somatomedin C) levels in patients with acromegaly.
Single doses of Sandostatin Injection given subcutaneously have been shown to inhibit gallbladder contractility and to decrease bile secretion in normal volunteers. In controlled clinical trials, the incidence of gallstone or biliary sludge formation was markedly increased [see Warnings and Precautions (5.1)].
Octreotide may cause clinically significant suppression of TSH.
12.3 Pharmacokinetics
Sandostatin Injection
According to data obtained with the immediate-release formulation, Sandostatin Injection solution, after subcutaneous injection, octreotide is absorbed rapidly and completely from the injection site. Peak concentrations of 5.2 ng/mL (100-mcg dose) were reached 0.4 hours after dosing. Using a specific radioimmunoassay, intravenous and subcutaneous doses were found to be bioequivalent. Peak concentrations and area under the curve (AUC) values were dose proportional both after subcutaneous or intravenous single doses up to 400 mcg and with multiple doses of 200 mcg 3 times daily (600 mcg/day). Clearance was reduced by about 66% suggesting nonlinear kinetics of the drug at daily doses of 600 mcg/day compared to 150 mcg/day. The relative decrease in clearance with doses above 600 mcg/day is not defined.
In healthy volunteers, the distribution of octreotide from plasma was rapid (tα1/2 = 0.2 h), the volume of distribution (Vdss) was estimated to be 13.6 L and the total body clearance was 10 L/h.
In blood, the distribution of octreotide into the erythrocytes was found to be negligible and about 65% was bound in the plasma in a concentration-independent manner. Binding was mainly to lipoprotein and, to a lesser extent, to albumin.
The elimination of octreotide from plasma had an apparent half-life of 1.7 hours, compared with the 1-3 minutes with the natural hormone, somatostatin. The duration of action of subcutaneously administered Sandostatin Injection solution is variable but extends up to 12 hours depending upon the type of tumor, necessitating multiple daily dosing with this immediate-release dosage form. About 32% of the dose is excreted unchanged into the urine. In an elderly population, dose adjustments may be necessary due to a significant increase in the half-life (46%) and a significant decrease in the clearance (26%) of the drug.
In patients with acromegaly, the pharmacokinetics differ somewhat from those in healthy volunteers. A mean peak concentration of 2.8 ng/mL (100-mcg dose) was reached in 0.7 hours after subcutaneous dosing. The Vdss was estimated to be 21.6 ± 8.5 L and the total body clearance was increased to 18 L/h. The mean percent of the drug bound was 41.2%. The disposition and elimination half-lives were similar to normals.
The half-life in renal-impaired patients was slightly longer than normal subjects (2.4-3.1 h versus 1.9 h). The clearance in renal-impaired patients was 7.3-8.8 L/h as compared to 8.3 L/h in healthy subjects. In patients with severe renal failure requiring dialysis, clearance was reduced to about half that found in healthy subjects (from approximately 10 L/h to 4.5 L/h).
Patients with liver cirrhosis showed prolonged elimination of drug, with octreotide half-life increasing to 3.7 h and total body clearance decreasing to 5.9 L/h, whereas patients with fatty liver disease showed half-life increasing to 3.4 h and total body clearance of 8.4 L/h. In normal subjects, octreotide half-life is 1.9 h and the clearance is 8.3 L/h which is comparable with the clearance in fatty-liver patients.
SANDOSTATIN LAR DEPOT
The magnitude and duration of octreotide serum concentrations after an intramuscular injection of the long-acting depot formulation SANDOSTATIN LAR DEPOT reflect the release of drug from the microsphere polymer matrix. Drug release is governed by the slow biodegration of the microspheres in the muscle, but once present in the systemic circulation, octreotide distributes and is eliminated according to its known pharmacokinetic properties which are as follows.
After a single IM injection of the long-acting depot dosage form SANDOSTATIN LAR DEPOT in healthy volunteer subjects, the serum octreotide concentration reached a transient initial peak of about 0.03 ng/mL/mg within 1 hour after administration progressively declining over the following 3-5 days to a nadir of < 0.01 ng/mL/mg, then slowly increasing and reaching a plateau about 2-3 weeks postinjection. Plateau concentrations were maintained over a period of nearly 2-3 weeks, showing dose proportional peak concentrations of about 0.07 ng/mL/mg. After about 6 weeks postinjection, octreotide concentration slowly decreased, to < 0.01 ng/mL/mg by Weeks 12 to 13, concomitant with the terminal degradation phase of the polymer matrix of the dosage form. The relative bioavailability of the long-acting release SANDOSTATIN LAR DEPOT compared to immediate-release Sandostatin Injection solution given subcutaneously was 60%-63%.
In patients with acromegaly, the octreotide concentrations after single doses of 10 mg, 20 mg, and 30 mg SANDOSTATIN LAR DEPOT were dose proportional. The transient Day 1 peak, amounting to 0.3 ng/mL, 0.8 ng/mL, and 1.3 ng/mL, respectively, was followed by plateau concentrations of 0.5 ng/mL, 1.3 ng/mL, and 2.0 ng/mL, respectively, achieved about 3 weeks postinjection. These plateau concentrations were maintained for nearly 2 weeks.
Following multiple doses of SANDOSTATIN LAR DEPOT given every 4 weeks, steady-state octreotide serum concentrations were achieved after the third injection. Concentrations were dose proportional and higher by a factor of approximately 1.6 to 2.0 compared to the concentrations after a single dose. The steady-state octreotide concentrations were 1.2 ng/mL and 2.1 ng/mL, respectively, at trough and 1.6 ng/mL and 2.6 ng/mL, respectively, at peak with 20 mg and 30 mg SANDOSTATIN LAR DEPOT given every 4 weeks. No accumulation of octreotide beyond that expected from the overlapping release profiles occurred over a duration of up to 28 monthly injections of SANDOSTATIN LAR DEPOT. With the long-acting depot formulation SANDOSTATIN LAR DEPOT administered IM every 4 weeks the peak-to-trough variation in octreotide concentrations ranged from 44%-68%, compared to the 163%-209% variation encountered with the daily subcutaneous three times daily regimen of Sandostatin Injection solution.
In patients with carcinoid tumors, the mean octreotide concentrations after 6 doses of 10 mg, 20 mg, and 30 mg SANDOSTATIN LAR DEPOT administered by IM injection every 4 weeks were 1.2 ng/mL, 2.5 ng/mL, and 4.2 ng/mL, respectively. Concentrations were dose proportional and steady-state concentrations were reached after 2 injections of 20 mg and 30 mg and after 3 injections of 10 mg.
SANDOSTATIN LAR DEPOT has not been studied in patients with renal impairment.
SANDOSTATIN LAR DEPOT has not been studied in patients with hepatic impairment.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in laboratory animals have demonstrated no mutagenic potential of Sandostatin. No mutagenic potential of the polymeric carrier in SANDOSTATIN LAR DEPOT, D,L-lactic and glycolic acids copolymer, was observed in the Ames mutagenicity test.
No carcinogenic potential was demonstrated in mice treated subcutaneously with octreotide for 85-99 weeks at doses up to 2000 mcg/kg/day (8 x the human exposure based on body surface area). In a 116-week subcutaneous study in rats administered octreotide, a 27% and 12% incidence of injection-site sarcomas or squamous cell carcinomas was observed in males and females, respectively, at the highest dose level of 1250 mcg/kg/day (10 x the human exposure based on body surface area) compared to an incidence of 8%-10% in the vehicle-control groups. The increased incidence of injection-site tumors was most probably caused by irritation and the high sensitivity of the rat to repeated subcutaneous injections at the same site. Rotating injection sites would prevent chronic irritation in humans. There have been no reports of injection-site tumors in patients treated with Sandostatin Injection for at least 5 years. There was also a 15% incidence of uterine adenocarcinomas in the 1250 mcg/kg/day females compared to 7% in the saline-control females and 0% in the vehicle-control females. The presence of endometritis coupled with the absence of corpora lutea, the reduction in mammary fibroadenomas, and the presence of uterine dilatation suggest that the uterine tumors were associated with estrogen dominance in the aged female rats which does not occur in humans.
Octreotide did not impair fertility in rats at doses up to 1000 mcg/kg/day, which represents 7 x the human exposure based on body surface area.
-
14 CLINICAL STUDIES
14.1 Acromegaly
The clinical trials of SANDOSTATIN LAR DEPOT were performed in patients who had been receiving Sandostatin Injection for a period of weeks to as long as 10 years. The acromegaly studies with SANDOSTATIN LAR DEPOT described below were performed in patients who achieved GH levels of < 10 ng/mL (and, in most cases < 5 ng/mL) while on subcutaneous Sandostatin Injection. However, some patients enrolled were partial responders to subcutaneous Sandostatin Injection, i.e., GH levels were reduced by > 50% on subcutaneous Sandostatin Injection compared to the untreated state, although not suppressed to < 5 ng/mL.
SANDOSTATIN LAR DEPOT was evaluated in three clinical trials in acromegalic patients.
In 2 of the clinical trials, a total of 101 patients were entered who had, in most cases, achieved a GH level < 5 ng/mL on Sandostatin Injection given in doses of 100 mcg or 200 mcg three times daily. Most patients were switched to 20 mg or 30 mg doses of SANDOSTATIN LAR DEPOT given once every 4 weeks for up to 27 to 28 injections. A few patients received doses of 10 mg and a few required doses of 40 mg. Growth hormone and IGF-1 levels were at least as well controlled with SANDOSTATIN LAR DEPOT as they had been on Sandostatin Injection and this level of control remained for the entire duration of the trials.
A third trial was a 12-month study that enrolled 151 patients who had a GH level < 10 ng/mL after treatment with Sandostatin Injection (most had levels < 5 ng/mL). The starting dose of SANDOSTATIN LAR DEPOT was 20 mg every 4 weeks for 3 doses. Thereafter, patients received 10 mg, 20 mg, or 30 mg every 4 weeks, depending upon the degree of GH suppression [see Dosage and Administration (2)]. Growth hormone and IGF-1 were at least as well controlled on SANDOSTATIN LAR DEPOT as they had been on Sandostatin Injection.
Table 5 summarizes the data on hormonal control (GH and IGF-1) for those patients in the first two clinical trials who received all 27 to 28 injections of SANDOSTATIN LAR DEPOT.
Table 5. Hormonal Response in Acromegalic Patients Receiving 27 to 28 Injections During1 Treatment With SANDOSTATIN LAR DEPOT 1Average of monthly levels of GH and IGF-1 over the course of the trials. Mean Hormone Level Sandostatin Injection S.C. SANDOSTATIN LAR DEPOT n % n % GH < 5.0 ng/mL 69/88 78 73/88 83 < 2.5 ng/mL 44/88 50 41/88 47 < 1.0 ng/mL 6/88 7 10/88 11 IGF-1 normalized 36/88 41 45/88 51 GH < 5.0 ng/mL + IGF-1 normalized 36/88 41 45/88 51 < 2.5 ng/mL + IGF-1 normalized 30/88 34 37/88 42 < 1.0 ng/mL + IGF-1 normalized 5/88 6 10/88 11 For the 88 patients in Table 5, a mean GH level of < 2.5 ng/mL was observed in 47% receiving SANDOSTATIN LAR DEPOT. Over the course of the trials, 42% of patients maintained mean growth hormone levels of < 2.5 ng/mL and mean normal IGF-1 levels.
Table 6 summarizes the data on hormonal control (GH and IGF-1) for those patients in the third clinical trial who received all 12 injections of SANDOSTATIN LAR DEPOT.
Table 6. Hormonal Response in Acromegalic Patients Receiving 12 Injections During1 Treatment With SANDOSTATIN LAR DEPOT 1Average of monthly levels of GH and IGF-1 over the course of the trial. Mean Hormone Level Sandostatin Injection S.C. SANDOSTATIN LAR DEPOT n % n % GH < 5.0 ng/mL 116/122 95 118/122 97 < 2.5 ng/mL 84/122 69 80/122 66 < 1.0 ng/mL 25/122 21 28/122 23 IGF-1 normalized 82/122 67 82/122 67 GH < 5.0 ng/mL + IGF-1 normalized 80/122 66 82/122 67 < 2.5 ng/mL + IGF-1 normalized 65/122 53 70/122 57 < 1.0 ng/mL + IGF-1 normalized 23/122 19 27/122 22 For the 122 patients in Table 6, who received all 12 injections in the third trial, a mean GH level of < 2.5 ng/mL was observed in 66% receiving SANDOSTATIN LAR DEPOT. Over the course of the trial, 57% of patients maintained mean growth hormone levels of < 2.5 ng/mL and mean normal IGF-1 levels. In comparing the hormonal response in these trials, note that a higher percentage of patients in the third trial suppressed their mean GH to < 5 ng/mL on subcutaneous Sandostatin Injection, 95%, compared to 78% across the 2 previous trials.
In all 3 trials, GH, IGF-1, and clinical symptoms were similarly controlled on SANDOSTATIN LAR DEPOT as they had been on Sandostatin Injection.
Of the 25 patients who completed the trials and were partial responders to Sandostatin Injection (GH > 5.0 ng/mL but reduced by > 50% relative to untreated levels), 1 patient (4%) responded to SANDOSTATIN LAR DEPOT with a reduction of GH to < 2.5 ng/mL and 8 patients (32%) responded with a reduction of GH to < 5.0 ng/mL.
Two open-label clinical studies investigated a 48-week treatment with SANDOSTATIN LAR DEPOT in 143 untreated (de novo) acromegalic patients. The median reduction in tumor volume was 20.6% in Study 1 (49 patients) at 24 weeks and 24.5% in Study 2 (94 patients) at 24 weeks and 36.2% at 48 weeks.
14.2 Carcinoid Syndrome
A 6-month clinical trial of malignant carcinoid syndrome was performed in 93 patients who had previously been shown to be responsive to Sandostatin Injection. Sixty-seven (67) patients were randomized at baseline to receive double-blind doses of 10 mg, 20 mg, or 30 mg SANDOSTATIN LAR DEPOT every 28 days and 26 patients continued, unblinded, on their previous Sandostatin Injection regimen (100-300 mcg three times daily).
In any given month after steady-state levels of octreotide were reached, approximately 35%-40% of the patients who received SANDOSTATIN LAR DEPOT required supplemental subcutaneous Sandostatin Injection therapy usually for a few days, to control exacerbation of carcinoid symptoms. In any given month, the percentage of patients randomized to subcutaneous Sandostatin Injection, who required supplemental treatment with an increased dose of Sandostatin Injection, was similar to the percentage of patients randomized to SANDOSTATIN LAR DEPOT. Over the 6-month treatment period, approximately 50%-70% of patients who completed the trial on SANDOSTATIN LAR DEPOT required subcutaneous Sandostatin Injection supplemental therapy to control exacerbation of carcinoid symptoms although steady-state serum SANDOSTATIN LAR DEPOT levels had been reached.
Table 7 presents the average number of daily stools and flushing episodes in malignant carcinoid patients.
Table 7. Average Number of Daily Stools and Flushing Episodes in Patients With Malignant Carcinoid Syndrome Treatment Daily Stools
(Average Number)Daily Flushing Episodes
(Average Number)n Baseline Last Visit Baseline Last Visit Sandostatin Injection S.C. 26 3.7 2.6 3.0 0.5 SANDOSTATIN LAR DEPOT 10 mg 22 4.6 2.8 3.0 0.9 20 mg 20 4.0 2.1 5.9 0.6 30 mg 24 4.9 2.8 6.1 1.0 Overall, mean daily stool frequency was as well controlled on SANDOSTATIN LAR DEPOT as on Sandostatin Injection (approximately 2-2.5 stools/day).
Mean daily flushing episodes were similar at all doses of SANDOSTATIN LAR DEPOT and on Sandostatin Injection (approximately 0.5-1 episode/day).
In a subset of patients with variable severity of disease, median 24 hour urinary 5-HIAA (5-hydroxyindole acetic acid) levels were reduced by 38%-50% in the groups randomized to SANDOSTATIN LAR DEPOT.
The reductions are within the range reported in the published literature for patients treated with octreotide (about 10%-50%).
Seventy-eight (78) patients with malignant carcinoid syndrome who had participated in this 6-month trial, subsequently participated in a 12-month extension study in which they received 12 injections of SANDOSTATIN LAR DEPOT at 4-week intervals. For those who remained in the extension trial, diarrhea and flushing were as well controlled as during the 6-month trial. Because malignant carcinoid disease is progressive, as expected, a number of deaths (8 patients: 10%) occurred due to disease progression or complications from the underlying disease. An additional 22% of patients prematurely discontinued SANDOSTATIN LAR DEPOT due to disease progression or worsening of carcinoid symptoms.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
SANDOSTATIN LAR DEPOT is available in single-use kits containing a 6-mL vial of 10 mg, 20 mg or 30 mg strength, a syringe containing 2 mL of diluent, one vial adapter, and one sterile 1½” 19 gauge safety injection needle. An instruction booklet for the preparation of drug suspension for injection is also included with each kit.
Drug Product Kits
10 mg kit NDC 0078-0811-81
20 mg kit NDC 0078-0818-81
30 mg kit NDC 0078-0825-81
Demonstration kit 0078-9825-81
For prolonged storage, SANDOSTATIN LAR DEPOT should be stored at refrigerated temperatures between 2°C to 8°C (36°F to 46°F) and protected from light until the time of use. SANDOSTATIN LAR DEPOT drug product kit should remain at room temperature for 30-60 minutes prior to preparation of the drug suspension. However, after preparation the drug suspension must be administered immediately.
-
17 PATIENT COUNSELING INFORMATION
Cholelithiasis and Complications of Cholelithiasis
Advise patients to contact their healthcare provider if they experience signs or symptoms of gallstones (cholelithiasis) or complications of cholelithiasis (e.g., cholecystitis, cholangitis and pancreatitis) [see Warnings and Precautions (5.1)].
Carcinoid Tumors and VIPomas
Patients with carcinoid tumors and VIPomas should be advised to adhere closely to their scheduled return visits for reinjection in order to minimize exacerbation of symptoms [see Dosage and Administration (2.2)].
Acromegaly
Patients with acromegaly should also be urged to adhere to their return visit schedule to help assure steady control of GH and IGF-1 levels [see Dosage and Administration (2.1)].
Pregnancy
Inform female patients that treatment with SANDOSTATIN LAR DEPOT may result in unintended pregnancy [see Use in Specific Populations (8.3)].
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936© Novartis
T2019-56
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 10 mg
Rx Only NDC: 0078-0646-81
Sandostatin® LAR Depot
(octreotide acetate for injectable suspension)
10 mg
For Intragluteal Injection

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 20 mg
Rx Only NDC: 0078-0647-81
Sandostatin® LAR Depot
(octreotide acetate for injectable suspension)
20 mg
For Intragluteal Injection

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 30 mg
Rx Only NDC: 0078-0648-81
Sandostatin® LAR Depot
(octreotide acetate for injectable suspension)
30 mg
For Intragluteal Injection

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 10 mg
Rx Only NDC: 0078-0811-81
Sandostatin® LAR Depot
(octreotide acetate for injectable suspension)
10 mg
For Intragluteal Injection

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 20 mg
Rx Only NDC: 0078-0818-81
Sandostatin® LAR Depot
(octreotide acetate for injectable suspension)
20 mg
For Intragluteal Injection

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 30 mg
Rx Only NDC: 0078-0825-81
Sandostatin® LAR Depot
(octreotide acetate for injectable suspension)
30 mg
For Intragluteal Injection

-
INGREDIENTS AND APPEARANCE
SANDOSTATIN LAR DEPOT
octreotide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0646 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0646-81 1 in 1 KIT 02/01/2015 11/30/2018 1 NDC: 0078-0646-61 1 in 1 VIAL, SINGLE-USE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SANDOSTATIN LAR DEPOT
octreotide acetate injection, powder, for suspensionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTREOTIDE ACETATE (UNII: 75R0U2568I) (OCTREOTIDE - UNII:RWM8CCW8GP) OCTREOTIDE 1.667 mg in 1 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID, DL- (UNII: 3B8D35Y7S4) 31.467 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6.833 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 11/30/2018 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 11/30/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 11/30/2018 SANDOSTATIN LAR DEPOT
octreotide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0647 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0647-81 1 in 1 KIT 02/01/2015 06/30/2019 1 NDC: 0078-0647-61 1 in 1 VIAL, SINGLE-USE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SANDOSTATIN LAR DEPOT
octreotide acetate injection, powder, for suspensionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTREOTIDE ACETATE (UNII: 75R0U2568I) (OCTREOTIDE - UNII:RWM8CCW8GP) OCTREOTIDE 3.33 mg in 1 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID, DL- (UNII: 3B8D35Y7S4) 62.933 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 13.65 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 06/30/2019 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 06/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 06/30/2019 SANDOSTATIN LAR DEPOT
octreotide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0648 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0648-81 1 in 1 KIT 02/01/2015 03/31/2019 1 NDC: 0078-0648-61 1 in 1 VIAL, SINGLE-USE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SANDOSTATIN LAR DEPOT
octreotide acetate injection, powder, for suspensionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTREOTIDE ACETATE (UNII: 75R0U2568I) (OCTREOTIDE - UNII:RWM8CCW8GP) OCTREOTIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID, DL- (UNII: 3B8D35Y7S4) 94.4 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 20.483 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 03/31/2019 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 03/31/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 02/01/2015 03/31/2019 SANDOSTATIN LAR DEPOT
octreotide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0811 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0811-81 1 in 1 KIT 07/22/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SANDOSTATIN LAR DEPOT
octreotide acetate injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0790 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTREOTIDE ACETATE (UNII: 75R0U2568I) (OCTREOTIDE - UNII:RWM8CCW8GP) OCTREOTIDE 1.667 mg in 1 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID, DL- (UNII: 3B8D35Y7S4) 31.467 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6.833 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0790-61 6 mL in 1 VIAL; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 SANDOSTATIN LAR DEPOT
octreotide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0818 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0818-81 1 in 1 KIT 07/22/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SANDOSTATIN LAR DEPOT
octreotide acetate injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0797 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTREOTIDE ACETATE (UNII: 75R0U2568I) (OCTREOTIDE - UNII:RWM8CCW8GP) OCTREOTIDE 3.33 mg in 1 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID, DL- (UNII: 3B8D35Y7S4) 62.933 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 13.65 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0797-61 6 mL in 1 VIAL; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 SANDOSTATIN LAR DEPOT
octreotide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0825 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0825-81 1 in 1 KIT 07/22/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SANDOSTATIN LAR DEPOT
octreotide acetate injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0804 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTREOTIDE ACETATE (UNII: 75R0U2568I) (OCTREOTIDE - UNII:RWM8CCW8GP) OCTREOTIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID, DL- (UNII: 3B8D35Y7S4) 94.4 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 20.483 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0804-61 6 mL in 1 VIAL; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 6 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021008 07/22/2016 Labeler - Novartis Pharmaceuticals Corporation (002147023)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.