TEXACLEAR ONE DOSE ALLERGY RELIEF
TEXACLEAR FAST ACTING ALLERGY RELIEF by
Drug Labeling and Warnings
TEXACLEAR FAST ACTING ALLERGY RELIEF by is a Otc medication manufactured, distributed, or labeled by GM Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TEXACLEAR FAST ACTING ALLERGY RELIEF- chlophedianol hydrochloride, pyrilamine maleate liquid
GM Pharmaceuticals, Inc.
----------
TEXACLEAR ONE DOSE ALLERGY RELIEF
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
- a cough that occurs with too much phlegm (mucus)
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if:
- nervousness, dizziness or sleeplessness occurs
- symptoms do not improve within 7 days, tends to recur, or are accompanied by a fever, rash or persistent headache. A persistent cough may be a sign of a serious condition.
- new symptoms occur
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
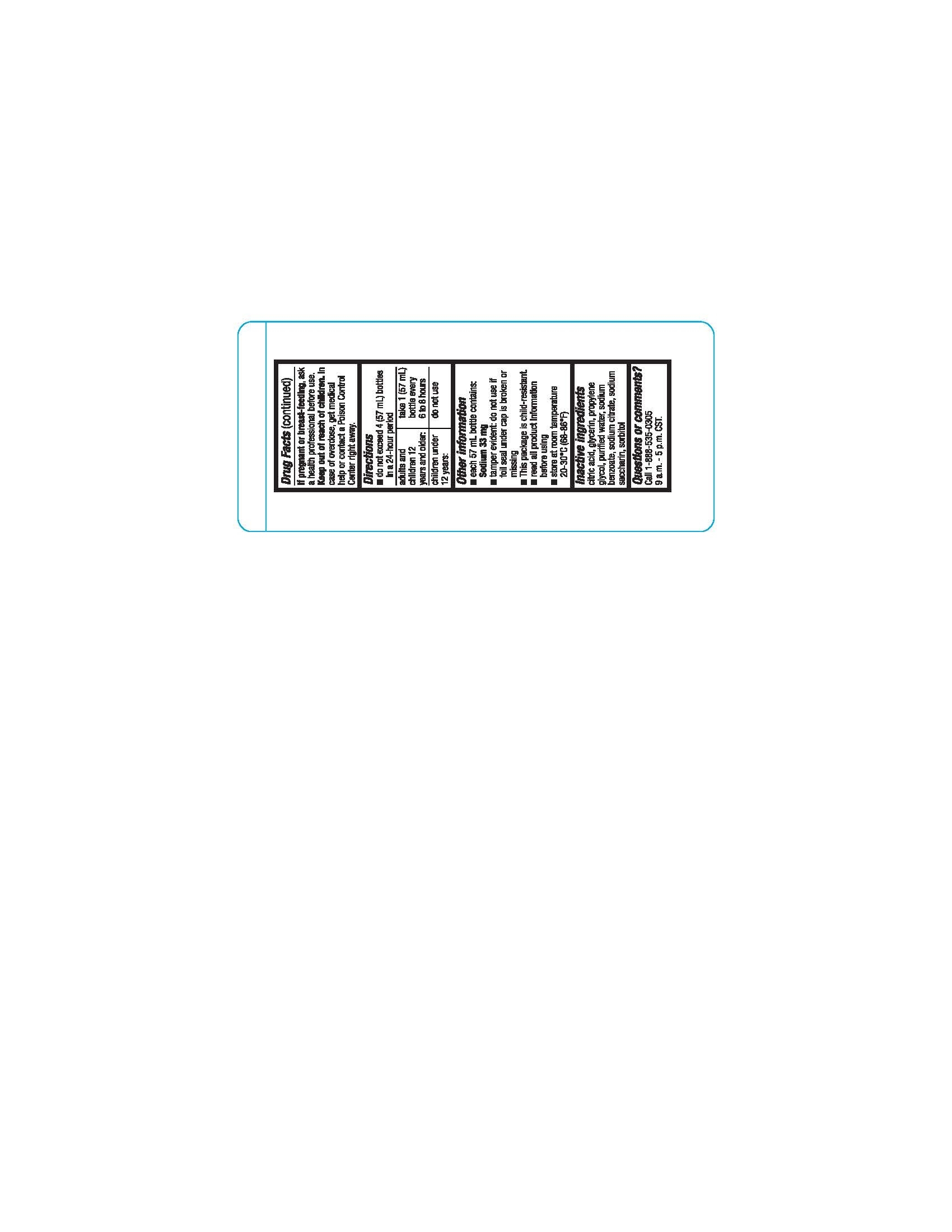
Directions
- Do not exceed 4 (57mL) bottles in a 24-hour period
|
Adults and children 12 years of age and over: |
Take 1 (57mL) bottle every 6 to 8 hours |
|
Children under 12 years of age: |
Do not use. |
Other information
- Each 57 mL bottle contains: Sodium 33 mg.
- tamper evident: Do not use if foil seal under cap is broken or missing.
- read all product information before using
- Store at 68° to 86°F (20° to 30°C).
- this package is child resistant
| TEXACLEAR FAST ACTING ALLERGY RELIEF
chlophedianol hydrochloride, pyrilamine maleate liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GM Pharmaceuticals, Inc. (793000860) |