Hy-Vee, Inc. Dayhist® Allergy Drug Facts
Dayhist allergy by
Drug Labeling and Warnings
Dayhist allergy by is a Otc medication manufactured, distributed, or labeled by HyVee Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DAYHIST ALLERGY- clemastine fumarate tablet
HyVee Inc
----------
Hy-Vee, Inc. Dayhist® Allergy Drug Facts
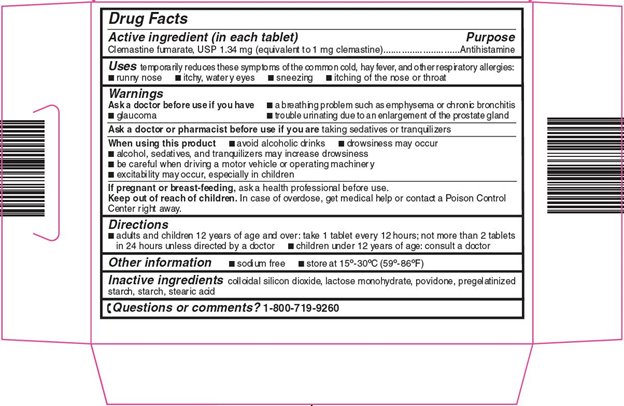
Uses
temporarily reduces these symptoms of the common cold, hay fever, and other respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlargement of the prostate gland
Directions
- adults and children 12 years of age and over: take 1 tablet every 12 hours; not more than 2 tablets in 24 hours unless directed by a doctor
- children under 12 years of age: consult a doctor
Inactive ingredients
colloidal silicon dioxide, lactose monohydrate, povidone, pregelatinized starch, starch, stearic acid
| DAYHIST ALLERGY
clemastine fumarate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - HyVee Inc (006925671) |
Revised: 11/2019
Document Id: dd0cf94a-a664-4aa9-9301-33c3fdb53fac
Set id: d0daae1c-f6fb-4a23-9aa9-0f68b1aea3c7
Version: 2
Effective Time: 20191115
HyVee Inc