NEOSTIGMINE METHYLSULFATE injection, solution
Neostigmine Methylsulfate by
Drug Labeling and Warnings
Neostigmine Methylsulfate by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NEOSTIGMINE METHYLSULFATE injection safely and effectively. See full prescribing information for NEOSTIGMINE METHYLSULFATE injection.
NEOSTIGMINE METHYLSULFATE injection, for intravenous use
Initial U.S. Approval: 1939INDICATIONS AND USAGE
Neostigmine Methylsulfate, a cholinesterase inhibitor, is indicated for reversal of the effects of nondepolarizing neuromuscular blocking agents (NMBA) after surgery ( 1).
DOSAGE AND ADMINISTRATION
Dosage
- Should be administered by trained healthcare providers ( 2.1)
- Recommend use of a peripheral nerve stimulator to determine whether neostigmine methylsulfate should be administered and to monitor recovery from neuromuscular blockade ( 2.1).
- Recommended dosage range is 0.03 mg/kg to 0.07 mg/kg for reversing non-depolarizing neuromuscular block when administered with an anticholinergic agent (atropine or glycopyrrolate) (
2.2,
2.3,
2.4)
- For reversal of NMBAs with shorter half-lives, when first twitch response is substantially greater than 10% of baseline, or when a second twitch is present: 0.03 mg/kg by intravenous route ( 2.2)
- For reversal of NMBAs with longer half-lives or when first twitch response is close to 10% of baseline: 0.07 mg/kg by intravenous route ( 2.2)
- Maximum total dosage is 0.07 mg/kg or up to a total of 5 mg (whichever is less) ( 2.2)
- An anticholinergic agent, e.g., atropine sulfate or glycopyrrolate, should be administered prior to or concomitantly with neostigmine methylsulfate ( 2.4)
Dose of Anticholinergic Agent (atropine or glycopyrrolate)
- Administer atropine sulfate (~15 mcg/kg) or glycopyrrolate (~10 mcg/kg) intravenously either several minutes before or concomitantly with neostigmine methylsulfate (using separate syringes) ( 2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 0.5 mg/mL and 1 mg/mL solution in 10 mL multiple dose vial in packages of 10 vials ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Bradycardia: Atropine or glycopyrrolate should be administered prior to administration of neostigmine methylsulfate injection to lessen risk of bradycardia ( 5.1)
- Coexisting Conditions: Patients with known cardiac disease, cardiac arrhythmias, or recent coronary artery occlusion may be particularly sensitive to the hemodynamic effects of neostigmine; their blood pressure and electrocardiogram should be continuously monitored with the initiation of neostigmine treatment and for a duration sufficient to assure hemodynamic stability. ( 5.2)
- Neuromuscular Dysfunction: Can occur if large doses of neostigmine methylsulfate are administered when there is minimal neuromuscular blockade; reduce the dose if recovery from neuromuscular blockade is nearly complete. ( 5.4)
ADVERSE REACTIONS
The most common adverse reactions include bradycardia and nausea and vomiting. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi, Vigilance & Medical Affairs at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: No human data and limited animal exist. Use only if clearly needed. (8)
Revised: 9/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Recommended Dosage in Adults
2.3 Recommended Dosage in Pediatric Patients, including Neonates
2.4 Concomitant or Pre-Administration of Anticholinergic Agents
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bradycardia
5.2 Cardiovascular Complications
5.3 Hypersensitivity (Anaphylaxis)
5.4 Neuromuscular Dysfunction
5.5 Cholinergic Crisis
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Depolarizing Muscle Relaxants
7.2 Antibiotics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- Neostigmine should be administered by trained healthcare providers familiar with the use, actions, characteristics, and complications of neuromuscular blocking agents (NMBA) and neuromuscular block reversal agents.
- Prior to Neostigmine Methylsulfate Injection administration and up until complete recovery of normal ventilation, the patient should be well ventilated and a patent airway maintained.
- Use a peripheral nerve stimulator capable of delivering a train-of-four (TOF) stimulus to evaluate the extent of recovery of neuromuscular function, and to determine the time of the first dose and the need for additional doses of Neostigmine Methylsulfate Injection.
- Prior to the administration of Neostigmine Methylsulfate Injection, there must be a twitch response to the first stimulus in the TOF of at least 10% of its baseline level (i.e., the response prior to NMBA administration).
- Dose selection should be based on the extent of spontaneous recovery at time of injection, half-life of the neuromuscular blocking agent (NMBA) to be reversed, and need for rapid NMBA reversal.
- Patients should continue to be monitored for adequacy of reversal of the effect of NMBAs for a period of time that would assure full recovery based on the patient’s medical condition and the pharmacokinetics of neostigmine and the NMBA used.
- Neostigmine Methylsulfate Injection is administered by intravenous bolus injection. Additional, carefully adjusted bolus doses are administered according to the patient’s response.
- An anticholinergic agent (e.g., atropine or glycopyrrolate) should be administered prior to or concomitantly with Neostigmine Methylsulfate Injection [ see Dosage and Administration ( 2.4), Warnings and Precautions ( 5.5) ].
- TOF monitoring alone should not be relied upon to determine the adequacy of reversal of neuromuscular blockade. Satisfactory recovery should be judged by the patient’s ability to maintain a patent airway, adequacy of ventilation, and skeletal muscle tone.
2.2 Recommended Dosage in Adults
- The recommended dose range of Neostigmine Methylsulfate Injection is 0.03 mg/kg to 0.07 mg/kg administered as an intravenous bolus.
- A dose less than 0.04 mg/kg is recommended for reversal of the effect of NMBAs with shorter half-lives (e.g., rocuronium), or when the first twitch response to the TOF stimulus is substantially greater than 10% of baseline, or when a second twitch is present.
- A dose of 0.07 mg/kg is recommended for the reversal of the effect of NMBAs with longer half-lives (e.g., vecuronium or pancuronium), or when first twitch response is not substantially greater than 10% of baseline, or if there is need for more rapid recovery.
- Additional doses may be required. The recommended maximum total dose is 0.07 mg/kg or up to a total of 5 mg, whichever is less.
2.3 Recommended Dosage in Pediatric Patients, including Neonates
Adult guidelines should be followed when Neostigmine Methylsulfate Injection is administered to pediatric patients. Pediatric patients require Neostigmine Methylsulfate Injection doses similar to those for adult patients.
2.4 Concomitant or Pre-Administration of Anticholinergic Agents
An anticholinergic agent (e.g., atropine sulfate or glycopyrrolate) should be administered intravenously several minutes prior to or with Neostigmine Methylsulfate Injection administration using separate syringes. For bradycardic patients, the anticholinergic agent should be administered prior to Neostigmine Methylsulfate Injection.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Neostigmine is contraindicated in patients with:
- known hypersensitivity to neostigmine methylsulfate (known hypersensitivity reactions have included urticaria, angioedema, erythema multiforme, generalized rash, facial swelling, peripheral edema, pyrexia, flushing, hypotension, bronchospasm, bradycardia and anaphylaxis).
- peritonitis or mechanical obstruction of the urinary or intestinal tracts.
-
5 WARNINGS AND PRECAUTIONS
5.1 Bradycardia
Neostigmine has been associated with bradycardia. An anticholinergic agent, (e.g., atropine sulfate or glycopyrrolate) should be administered prior to Neostigmine Methylsulfate Injection administration to lessen the risk of bradycardia [ see Dosage and Administration ( 2.4) ].
5.2 Cardiovascular Complications
Cardiac arrhythmias, nonspecific electrocardiogram changes, cardiac arrest, syncope and hypotension have been reported with neostigmine methylsulfate. In patients with certain cardiovascular conditions such as coronary artery disease, cardiac arrhythmias or recent acute coronary syndrome, the risk of blood pressure and heart rate complications may be increased. Risk of these complications may also be increased in patients with myasthenia gravis. Standard antagonism with anticholinergics (e.g., atropine) is generally successful to mitigate the risk of cardiovascular complications.
5.3 Hypersensitivity (Anaphylaxis)
Hypersensitivity reactions including anaphylaxis have been reported with neostigmine. Ensure that appropriate medical support measures, including atropine, cardiopulmonary resuscitation equipment, and medications to treat anaphylaxis are readily available.
5.4 Neuromuscular Dysfunction
Neuromuscular dysfunction has been associated with administration of large doses of neostigmine when neuromuscular blockade is minimal. To mitigate the risk of neuromuscular dysfunction, consider reducing the dose of neostigmine if recovery from neuromuscular blockade is nearly complete.
5.5 Cholinergic Crisis
Overdosage of neostigmine may cause cholinesterase inhibitor toxicity or cholinergic crisis which may be difficult to differentiate from myasthenia crisis since both conditions present with similar symptoms. Both conditions result in extreme muscle weakness but require radically different treatments. Cholinergic crisis requires immediate withdrawal of all anticholinergic medication and immediate use of atropine [ see Overdosage ( 10) ].
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following serious adverse reactions are described below and elsewhere in the labeling:
- Bradycardia [ see Warnings and Precautions ( 5.1) ]
- Cardiovascular Complications [ see Warnings and Precautions ( 5.2) ]
- Hypersensitivity (Anaphylaxis) [ see Warnings and Precautions ( 5.3) ]
Adverse reactions to neostigmine methylsulfate are most often attributable to exaggerated pharmacological effects, in particular, at muscarinic receptor sites. The use of an anticholinergic agent, e.g., atropine sulfate or glycopyrrolate, may prevent or mitigate these reactions.
Quantitative adverse event data are available from trials of neostigmine methylsulfate in which 200 adult patients were exposed to the product. Adverse reactions that occurred with an overall frequency of 1% or greater included the following:
Allergic: Allergic reactions and anaphylaxis.
Neurological: Dizziness, syncope, weakness, convulsions, loss of consciousness, drowsiness, headache, dysarthria, miosis and visual changes.
Cardiovascular: Cardiac arrhythmias including bradycardia, tachycardia, atrioventricular block and nodal rhythm, as well as cardiac arrest, and hypotension.
Respiratory: Increased oral, pharyngeal and bronchial secretions, dyspnea, respiratory depression, oxygen desaturation, respiratory arrest and bronchospasm.
Dermatologic: Diaphoresis, flushing, rash, pruritus, and urticaria.
Gastrointestinal: Dry mouth, nausea, emesis, flatulence and increased peristalsis.
Genitourinary: Increased urinary frequency.
Musculoskeletal: Muscle cramps and spasm, arthralgia.
Psychiatric: Insomnia.
General: Incision site complication, pharyngolaryngeal pain, procedural complication, procedural pain.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during parenteral use of neostigmine methylsulfate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic Disorders: Allergic reactions, anaphylaxis
Nervous System Disorders: Convulsions, drowsiness, dysarthria, fasciculation, loss of consciousness, miosis, visual changes
Cardiovascular Disorders: Cardiac arrest, cardiac arrhythmias (A-V block, nodal rhythm), hypotension, nonspecific EKG changes, syncope
Respiratory, Thoracic and Mediastinal Disorders: Bronchospasm; increased oral, pharyngeal and bronchial secretions; respiratory arrest; respiratory depression
Skin and Subcutaneous Tissue Disorders: Rash, urticaria diaphoresis, flushing
Gastrointestinal Disorders: Bowel cramps, diarrhea, flatulence, increased peristalsis
Renal and Urinary Disorders: Urinary frequency
Musculoskeletal and Connective Tissue Disorders: Arthralgia, muscle cramps, spasms, weakness
-
7 DRUG INTERACTIONS
The pharmacokinetic interaction between neostigmine methylsulfate and other drugs has not been studied. Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver. Closely monitor patients for a longer period of time when using Neostigmine Methylsulfate Injection with other drugs which may alter the activity of metabolizing enzymes or transporters.
7.1 Depolarizing Muscle Relaxants
Use of neostigmine to reverse the effects of depolarizing muscle relaxants such as succinylcholine is not recommended, because it may prolong the phase-1 block.
7.2 Antibiotics
Certain antibiotics, particularly neomycin, streptomycin and kanamycin have nondepolarizing neuromuscular blocking action, and therefore, neostigmine dose adjustments may be required to reverse neuromuscular block in patients who have been taking these drugs. There was no effect on neostigmine action on rocuronium reversal by cefuroxime, metronidazole, cefuroxime or metronidazole.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate or well-controlled studies of Neostigmine Methylsulfate Injection in pregnant women. It is not known whether Neostigmine Methylsulfate Injection can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. The incidence of malformations in human pregnancies has not been established for neostigmine as the data are limited. All pregnancies, regardless of drug exposure, have a background risk of 2 to 4% for major birth defects, and 15 to 20% for pregnancy loss.
No adverse effects were noted in rats or rabbits treated with human equivalent doses of neostigmine methylsulfate doses up to 8.1 and 13 mcg/kg/day, respectively, during organogenesis (0.1 to 0.2-times the maximum recommended human dose of 5 mg/60 kg person/day based on body surface area comparisons).
Anticholinesterase drugs, including neostigmine may cause uterine irritability and induce premature labor when administered to pregnant women near term.
Neostigmine Methylsulfate Injection should be given to a pregnant woman only if clearly needed.
Data
Animal Data
In embryofetal development studies, rats and rabbits were administered neostigmine methylsulfate at human equivalent doses (HED, on a mg/m 2 basis) of 1.6, 4 and 8.1 mcg/kg/day 3.2, 8.1, and
13 mcg/kg/day, respectively, during the period of organogenesis (Gestation Days 6 through 17 for rats and Gestation Days 6 through 18 for rabbits). There was no evidence for a teratogenic effect in rats and rabbits up to HED 8.1 and 13 mcg/kg/day, which are approximately 0.097-times and 0.16-times the MRHD of 5 mg/60 kg, respectively in the presence of minimal maternal toxicity (tremors, ataxia, and prostration). The studies resulted in exposures in the animals well below predicted exposures in humans.
In a pre- and postnatal development study in rats, neostigmine methylsulfate was administered to pregnant female rats at human equivalent doses (HED) of 1.6, 4 and 8.1 mcg/kg/day from Day 6 of gestation through Day 20 of lactation, with weaning on Day 21. There were no adverse effects on physical development, behavior, learning ability, or fertility in the offspring occurred at HED doses up 8.1 mcg/kg/day which is 0.097-times the MRHD of 5 mg/60 kg on a mg/m 2 basis in the presence of minimal maternal toxicity (tremors, ataxia, and prostration). The studies resulted in exposures in the animals well below predicted exposures in humans.
8.2 Lactation
It is not known whether Neostigmine Methylsulfate Injection is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from Neostigmine Methylsulfate Injection in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Data from published literature support the intravenous use of neostigmine methylsulfate for reversal of nondepolarizing neuromuscular blocking agents in all pediatric age groups.
Recovery of neuromuscular activity occurs more rapidly with smaller doses of cholinesterase inhibitors in infants and children than in adults. However, infants and small children may be at greater risk of complications from incomplete reversal of neuromuscular blockade due to decreased respiratory reserve. The risks associated with incomplete reversal outweigh any risk from giving higher doses of Neostigmine Methylsulfate (up to 0.07 mg/kg or up to a total of 5 mg, whichever is lower).
The dose of Neostigmine Methylsulfate required to reverse neuromuscular blockade in children varies between 0.03 mg to 0.07 mg/kg, the same dose range shown to be effective in adults, and should be selected using the same criteria as used for adult patients [ see Clinical Pharmacology (12.3)].
Since the blood pressure in pediatric patients, particularly infants and neonates is sensitive to changes in heart rate, the effects of an anticholinergic agent (e.g., atropine) should be observed prior to administration of neostigmine to lessen the probability of bradycardia and hypotension.
8.5 Geriatric Use
Elderly patients are likely to have decreased renal function, which may prolong the duration of action of neostigmine methylsulfate. However, elderly patients also experience slower spontaneous recovery from neuromuscular blocking agents. Therefore, dosage adjustments are generally not needed in geriatric patients; however, they should be monitored for longer periods than younger adults to assure additional doses of Neostigmine Methylsulfate Injection are not required. The duration of monitoring should be predicated on the anticipated duration of action for the neuromuscular blocking agents used on the patient.
8.6 Renal Impairment
Elimination half-life of neostigmine was prolonged in anephric patients compared to normal subjects, so neostigmine concentration may increase in patients with impaired renal functions. Although no adjustments to Neostigmine Methylsulfate Injection dosing appear to be warranted in patients with impaired renal function, they should be closely monitored for a longer period of time.
To assure the effects of the neuromuscular blocking agent, particularly one cleared by the kidneys, do not persist beyond those of Neostigmine Methylsulfate Injection, the interval for re‐dosing the neuromuscular blocking agent during the surgical procedure may be useful in determining whether, and to what extent, post‐operative monitoring needs to be extended.
8.7 Hepatic Impairment
The pharmacokinetics of neostigmine methylsulfate in patients with hepatic impairment have not been studied. Neostigmine is metabolized by microsomal enzymes in the liver so neostigmine concentration may increase in patients with impaired hepatic functions. Although no adjustments to the dosing of Neostigmine Methylsulfate Injection appear to be warranted in patients with hepatic insufficiency, patients should be carefully monitored for a longer period of time.
If hepatically cleared neuromuscular blocking agents were used during the surgical procedure, their duration of action may also be prolonged by hepatic insufficiency. This could result in the effects of the neuromuscular blocking agent outlasting those of Neostigmine Methylsulfate Injection. In this regard, the interval for re-dosing the neuromuscular blocking agent during the surgical procedure may be useful in determining whether, and to what extent, post-operative monitoring needs to be extended.
-
10 OVERDOSAGE
Muscarinic symptoms (nausea, vomiting, diarrhea, sweating, increased bronchial and salivary secretions, and bradycardia) may appear with overdosage of neostigmine methylsulfate, but may be managed by the use of additional atropine or glycopyrrolate. The possibility of iatrogenic overdose can be lessened by carefully monitoring the muscle twitch response to peripheral nerve stimulation. Should overdosage occur, ventilation should be supported by artificial means until the adequacy of spontaneous respiration is assured, and cardiac function should be monitored.
Overdosage of neostigmine may also cause a cholinergic crisis, which is characterized by increasing muscle weakness, and through involvement of the muscles of respiration, may result in death if not promptly treated.
The treatment of an overdose of neostigmine includes immediate withdrawal of all anticholinergic medication and immediate use of atropine is also recommended. Assistance of ventilation may be required if respiration is severely depressed.
Myasthenic crisis, due to an increase in the severity of the disease, is also accompanied by extreme muscle weakness and may be difficult to distinguish from cholinergic crisis on a symptomatic basis. However, such differentiation is extremely important because increases in the dose of neostigmine methylsulfate or other drugs in this class, in the presence of cholinergic crisis or of a refractory or “insensitive” state, could have grave consequences. The two types of crises may be differentiated by the use of edrophonium chloride as well as by clinical judgment. Treatment of the two conditions differs radically. Whereas the presence of myasthenic crisis requires more intensive anticholinesterase therapy, cholinergic crisis calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended. Atropine may also be used to lessen gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
-
11 DESCRIPTION
Neostigmine Methylsulfate Injection, a cholinesterase inhibitor, has an empirical formula of C 13H 22N 2O 6S, a molecular weight of 334.39 g/mol and the following structural formula:
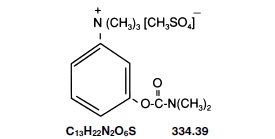
Neostigmine Methylsulfate Injection is formulated with neostigmine methylsulfate, a white crystalline powder, chemically designated as (m-hydroxyphenyl) trimethylammonium methylsulfate dimethylcarbamate.
Neostigmine Methylsulfate Injection is available in two dosage strengths; 0.5 mg/mL, and 1 mg/mL in 10 mL multiple dose amber glass vials.The composition per mL is as follows:
Ingredients
mg/mL
Neostigmine Methylsulfate
0.5
1
Phenol (as Liquefied Phenol, USP)
4.5
4.5
Sodium Acetate, USP (Trihydrate)
0.2
0.2
Water for Injection
q.s.
q.s
Phenol is added as a preservative. Acetic acid and/or sodium hydroxide may have been added for pH adjustment.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Neostigmine methylsulfate is a competitive cholinesterase inhibitor. By reducing the breakdown of acetylcholine, neostigmine methylsulfate induces an increase in acetylcholine in the synaptic cleft which competes for the same binding site as nondepolarizing neuromuscular blocking agents, and reverses the neuromuscular blockade.
12.2 Pharmacodynamics
Neostigmine is an anticholinesterase agent, and inhibits the hydrolysis of acetylcholine by competing with acetylcholine for binding to acetylcholinesterase at sites of cholinergic transmission. By reducing the breakdown of acetylcholine, neuromuscular transmission is facilitated. Neostigmine also has direct postsynaptic cholinomimetic effects which can be managed clinically by the co-administration of atropine or glycopyrrolate.
12.3 Pharmacokinetics
Distribution
Protein binding of neostigmine to human serum albumin ranges from 15 to 25%. The observed volume of distribution is between 0.12 and 1.4 L/kg following intravenous injection.
Elimination
Neostigmine is metabolized by microsomal enzymes in the liver and the observed elimination half-life reported is between 24 and 113 minutes.
Metabolism
Neostigmine is metabolized by microsomal enzymes in the liver.
Excretion
The observed elimination half-life reported is between 24 and 113 minutes following intravenous injection.
Specific Populations
Pediatric Population:
After intravenous administration as a 2-minute infusion (infants 2 to 10 months old: 100 mcg/kg; children 1 to 6 years old: 70 mcg/kg), the elimination half-life for infants and children were 39 ± 5 min and 48 ± 16 min (mean ± SD), respectively. Clearance for infants and children were 13.6 ± 2.8 and 11.1 ± 2.7 mL/min/kg (mean ± SD), respectively.
Renal Impairment
Elimination half-life was prolonged in anephric patients compared to normal subjects; elimination half-life for normal, transplant and anephric patients were 79.8 ± 48.6, 104.7 ± 64 and 181 ± 54 min (mean ± SD), respectively.
Hepatic Impairment
The pharmacokinetics of neostigmine in patients with hepatic impairment has not been studied. Neostigmine is metabolized by microsomal enzymes in the liver and its concentration may increase in patients with impaired hepatic functions.
Drug Interactions
The pharmacokinetic interaction between neostigmine and other drugs has not been studied. Neostigmine concentration may increase or decrease if concomitantly used drugs inhibit or induce the activity of metabolizing enzymes or transporters, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:
Long-term animal studies have not been performed to evaluate the carcinogenic potential of neostigmine methylsulfate.
Mutagenesis:
Neostigmine Methylsulfate Injection was not genotoxic in the in vitro bacterial reverse mutation assay (Ames test), in the in vitro chromosome aberration assay, or the in vivo rat micronucleus assay.
Impairment of Fertility:
In a fertility and early embryonic development study in rats, male rats were treated for 28 days prior to mating and female rats were treated for 14 days prior to mating with intravenous neostigmine methylsulfate (human equivalent doses of 1.6, 4, and 8.1 mcg/kg/day, based on body surface area). No adverse effects were reported at any dose (up to 0.1-times the MRHD of 5 mg/60 kg person based on a body surface area comparison).
-
14 CLINICAL STUDIES
Data from published literature support the intravenous use of neostigmine methylsulfate for reversal of nondepolarizing neuromuscular blocking agents. Randomized, spontaneous-recovery-controlled or placebo-controlled studies using similar efficacy endpoints evaluated a total of 404 adult and 80 pediatric patients undergoing various surgical procedures. Patients had reductions in their recovery time from neuromuscular blockade with neostigmine methylsulfate treatment compared to spontaneous recovery and placebo treatments.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Neostigmine Methylsulfate Injection is available in 10 mL multiple dose amber glass vials containing a clear, colorless solution of 0.5 mg per mL (NDC: 63323-413-36) or 1 mg per mL (NDC: 63323-415-36) of neostigmine methylsulfate injection supplied in packages of 10 vials.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature] and protect from light. Store vials in tray until ready for use.
The container closure is not made with natural rubber latex.
 is a registered trademark of Premier, Inc., used under license.
is a registered trademark of Premier, Inc., used under license.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY - Neostigmine 10 mL Vial Label
Neostigmine Methylsulfate Injection, USP
5 mg per 10 mL
(0.5 mg per mL)
For intravenous use.
10 mL Multiple Dose Vial Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Neostigmine 10 mL Vial Tray Label
Neostigmine Methylsulfate Injection, USP
5 mg per 10 mL
(0.5 mg per mL)
For intravenous use.
10 x 10 mL
Multiple Dose Vials Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Neostigmine 10 mL Vial Label
Neostigmine Methylsulfate Injection, USP
10 mg per 10 mL
(1 mg per mL)
For intravenous use.
10 mL Multiple Dose Vial Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Neostigmine 10 mL Vial Tray Label
Neostigmine Methylsulfate Injection, USP
10 mg per 10 mL
(1 mg per mL)
For intravenous use.
10 x 10 mL
Multiple Dose Vials Rx only

-
INGREDIENTS AND APPEARANCE
NEOSTIGMINE METHYLSULFATE
neostigmine methylsulfate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-413 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOSTIGMINE METHYLSULFATE (UNII: 98IMH7M386) (NEOSTIGMINE - UNII:3982TWQ96G) NEOSTIGMINE METHYLSULFATE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength PHENOL (UNII: 339NCG44TV) 4.5 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 0.2 mg in 1 mL ACETIC ACID (UNII: Q40Q9N063P) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-413-36 10 in 1 TRAY 01/08/2015 1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203629 01/08/2015 NEOSTIGMINE METHYLSULFATE
neostigmine methylsulfate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-415 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOSTIGMINE METHYLSULFATE (UNII: 98IMH7M386) (NEOSTIGMINE - UNII:3982TWQ96G) NEOSTIGMINE METHYLSULFATE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength PHENOL (UNII: 339NCG44TV) 4.5 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 0.2 mg in 1 mL ACETIC ACID (UNII: Q40Q9N063P) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-415-36 10 in 1 TRAY 01/08/2015 1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203629 01/08/2015 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 023648251 manufacture(63323-413, 63323-415) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 840771732 manufacture(63323-413, 63323-415)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.