McKesson Alcohol Prep Pad by McKesson Medical-Surgical
McKesson Alcohol Prep Pad by
Drug Labeling and Warnings
McKesson Alcohol Prep Pad by is a Otc medication manufactured, distributed, or labeled by McKesson Medical-Surgical. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MCKESSON ALCOHOL PREP PAD- isopropyl alcohol swab
McKesson Medical-Surgical
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
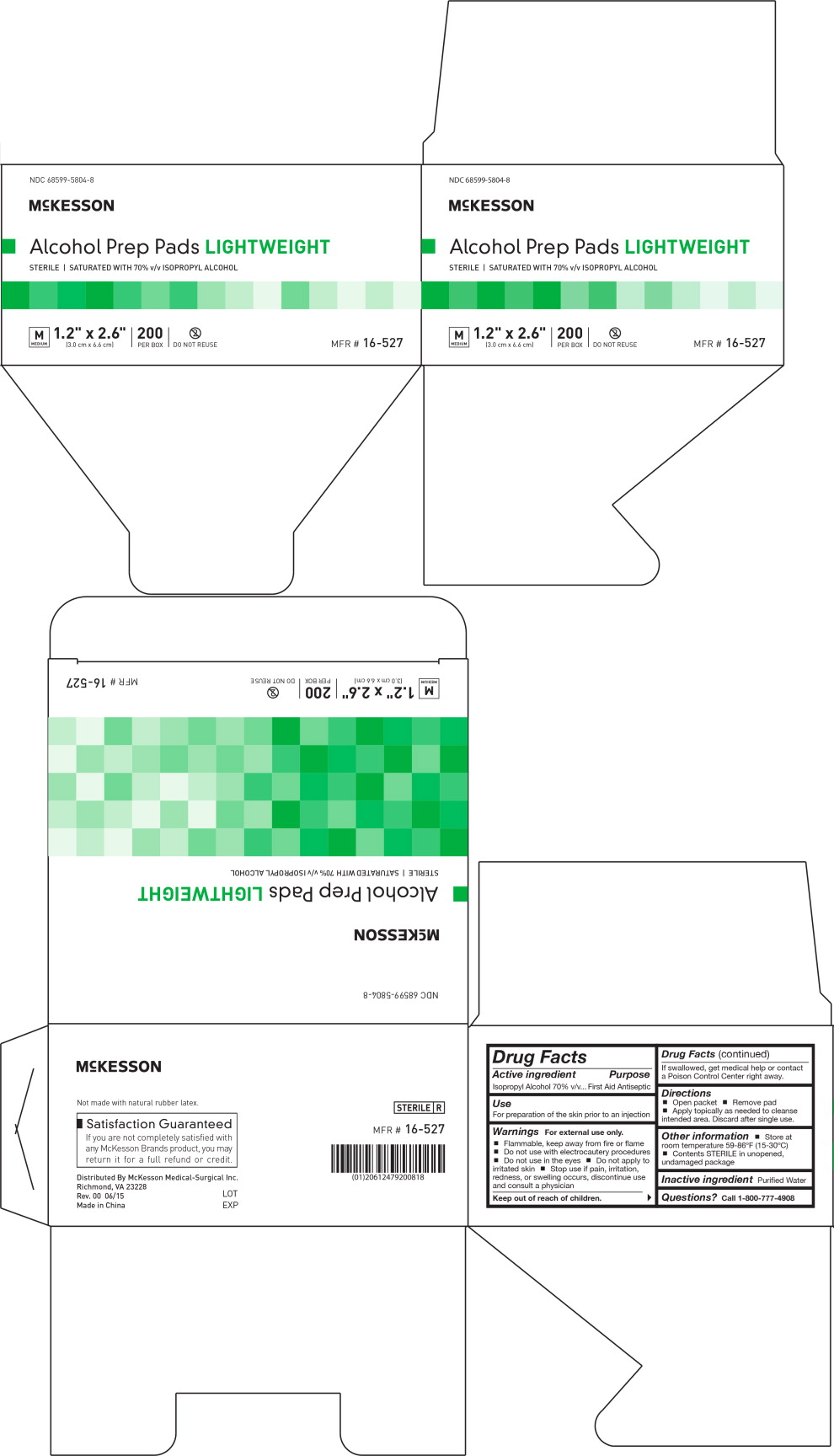
Warnings
For external use only.
- Flammable, keep away from fire or flame
- Do not use with electrocautery procedures
- Do not use in the eyes
- Do not apply to irritated skin
- Stop use if pain, irritation, redness, or swelling occurs, discontinue use and consult a physician
Directions
- Open packet
- Remove pad
- Apply topically as needed to cleanse intended area. Discard after single use.
Other information
- Store at room temperature 59-86°F (15-30°C)
- Contents STERILE in unopened, undamaged package
Principal Display Panel - Carton Label
NDC: 68599-5804-8
MCKESSON
Alcohol Prep Pads LIGHTWEIGHT
STERILE | SATURATED WITH 70% v/v ISOPROPYL ALCOHOL
M
MEDIUM
1.2" x 2.6"
(3.0 cm x 6.6 cm)
200
PER BOX
DO NO REUSE
MFR # 16-527
Principal Display Panel - Packet Label
NDC: 68599-5804-7
MCKESSON
Alcohol Prep Pad
LIGHTWEIGHT
STERILE | SATURATED WITH
70% v/v ISOPROPYL ALCOHOL
M
MEDIUM
1.2" x 2.6"
(3.0 cm x 6.6 cm)
DO NOT
REUSE
MFR # 16-527
Principal Display Panel - Carton Label
NDC: 68599-5804-0
MCKESSON
Alcohol Prep Pads
LIGHTWEIGHT
STERILE | SATURATED WITH 70% v/v ISOPROPYL ALCOHOL
L
LARGE
1.8" x 3.3"
(4.5 cm x 8.3 cm)
100
PER BOX
DO NO REUSE
MFR # 16-528
Principal Display Panel - Packet Label
NDC: 68599-5804-9
MCKESSON
Alcohol Prep Pad
LIGHTWEIGHT
STERILE | SATURATED WITH
70% v/v ISOPROPYL ALCOHOL
L
LARGE
1.8" x 3.3"
(4.5 cm x 8.3 cm)
DO NOT
REUSE
MFR # 16-528
| MCKESSON ALCOHOL PREP PAD
isopropyl alcohol swab |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - McKesson Medical-Surgical (023904428) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jiangsu Province Tech (Shanghai) | 530968767 | manufacture(68599-5804) | |