SODIUM CHLORIDE injection, solution
sodium chloride by
Drug Labeling and Warnings
sodium chloride by is a Prescription medication manufactured, distributed, or labeled by Medefil, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SODIUM CHLORIDE INJECTION, USP, 0.9% safely and effectively. See full prescribing information for SODIUM CHLORIDE INJECTION, USP, 0.9%.
SODIUM CHLORIDE INJECTION, USP, 0.9%
Initial U.S. Approval: 2012INDICATIONS AND USAGE
Sodium Chloride Injection, USP, 0.9% is indicated for:
Dilution or Dissolving the drugs for intravenous, intramuscular or subcutaneous injections ( 1.1).
DOSAGE AND ADMINISTRATION
Volume of preparation to be used for diluting or dissolving any drug for injection is dependent on the vehicle concentration, dose and route of administration recommended by the drug manufacturer ( 2.1).
DOSAGE FORMS AND STRENGTHS
Injection, 0.9% (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
Consult the drug product manufacturer’s instructions for choice of vehicle, appropriate dilution or volume for dissolving drugs to be injected including the route and rate of injection ( 5.1).
Do not use Sodium Chloride Injection, USP, 0.9% if the solution is discolored, cloudy, hazy, or contains a precipitate, or if the syringe is damaged ( 5.1).
For single use only. Discard unused portion ( 5.2).
ADVERSE REACTIONS
Reactions that may occur because of this solution, added drugs or the technique of reconstitution or administration include air embolization, febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasations ( 6).
To report SUSPECTED ADVERSE REACTIONS, contact MEDEFIL, INC. at tel:1-630-682-4600 and http://www.medefilinc.com/ or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Some drugs or injections may be incompatible when combined with 0.9% sodium chloride.
Consult with a pharmacist, if unsure of compatibility ( 7).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
1.1 Dilution or Dissolution of Drugs
2 DOSAGE & ADMINISTRATION
2.1 Recommended Dosage
2.2 Instruction for Administration
2.3 Preparation and Handling Precautions
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 General
5.2 For Single Use Only
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS & USAGE
-
2 DOSAGE & ADMINISTRATION
2.1 Recommended Dosage
- The volume of preparation to be used for diluting or dissolving any drug for injection is dependent on the vehicle concentration, dose and route of administration as recommended by the drug manufacturer.
2.2 Instruction for Administration
- Solution and fluid path are sterile and non-pyrogenic if the tip cap is in place, syringe is intact and there is no evidence of leakage. Use proper aseptic technique.
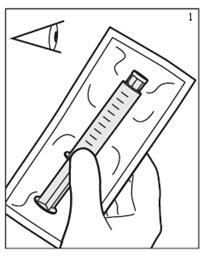
- Inspect plastic wrapping. Do not use if plastic wrapping is damaged or not intact. (Figure 1)
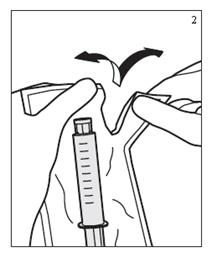
- Remove plastic packaging by tearing along perforation. (Figure 2)
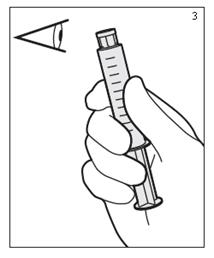
- Do not use if, solution is discolored, cloudy, hazy, or contains a precipitate, or if the syringe is damaged. (Figure 3)
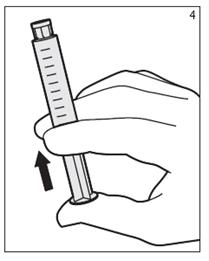
- With the tip cap of the syringe on, press the syringe forward to properly activate the syringe. Improper activation may difficult syringe use and/or may cause plunger rod separation. Never draw back rod because the product may become contaminated. (Figure 4)
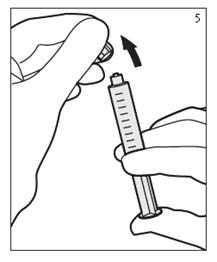
- Remove tip cap. Hold the syringe unit upright and prime to expel any air bubble if present. (Figure 5)
- Syringe is now ready to use.
- Use aseptic techniques for diluting or dissolving drugs, mix thoroughly and use according to the drug manufacturer’s label instructions.
- Discard empty unit after use. Discard any unused portion. Do not reuse disposable syringes.
2.3 Preparation and Handling Precautions
- Before use check for any possible incompatibility with sodium chloride.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Do not administer unless solution is clear and seal is intact.
- Do not store reconstituted solution or drugs for injection unless otherwise directed by the manufacturer of the solute.
- Adding additives may be incompatible.
- Do not use if, solution is discolored, cloudy, hazy, or contains a precipitate, or if the syringe is damaged.
- Do not freeze.
- Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat.
- 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 General
When used to dilute drug products, consult the drug product manufacturer’s instructions for choice of vehicle, appropriate dilution or volume for dissolving drugs to be injected including the route and rate of injection. Inspect reconstituted (diluted or dissolved) drugs for clarity (if soluble) and freedom from unexpected precipitation or discoloration prior to administration. Do not use Sodium Chloride Injection, USP 0.9% if the solution is discolored, cloudy, hazy, or contains a precipitate, or if the syringe is damaged.
-
6 ADVERSE REACTIONS
Adverse reactions which may occur because of this solution, added drugs or the technique of reconstitution or administration include, but are not limited to, air embolization, febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasation.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and if possible, retrieve and save the remainder of unused vehicle for examination if deemed necessary.
-
7 DRUG INTERACTIONS
Some drugs or injections may be incompatible when combined with 0.9% sodium chloride. Before Sodium Chloride Injection, USP, 0.9% is used as a vehicle for the administration of a drug, the drug product manufacturer’s instructions or other specific references should be checked for any possible incompatibility with sodium chloride.
Consult with a pharmacist, if unsure of compatibility.
-
11 DESCRIPTION
Sodium Chloride Injection, USP 0.9% is a sterile, nonpyrogenic, isotonic solution of sodium chloride and water for injection. It contains no bacteriostatic antimicrobial agents or added buffer. The nominal pH is 5.5 (4.5 to 7.0).
Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. Sodium Chloride Injection, USP, 0.9% contains 9 g/L Sodium Chloride, USP (NaCl) with a calculated osmolarity of approximately 308 mOsmol/L. It contains 154 mEq/L sodium and 154 mEq/L chloride.
The syringe component of Sodium Chloride Injection, USP, 0.9% is manufactured with polypropylene and luer lock.
The syringe is not made with natural rubber latex. The syringe is not made with DEHP. This product contains no preservative. The syringes require no vapor barrier to maintain the proper drug concentration. The empirical formula for sodium chloride is NaCl and the molecular weight is 58.44 g/mol.
Supplied as single use syringes.
-
12 CLINICAL PHARMACOLOGY
Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl-) ions. These ions are normal constituents of the body fluids (principally extracellular) and are essential for maintaining electrolyte balance.
The small volume of fluid and amount of sodium chloride provided by Sodium Chloride Injection, USP, 0.9% when used only as an isotonic vehicle for parenteral injection of drugs or for flushing of indwelling access devices, is unlikely to exert a significant effect on fluid and electrolyte balance except possibly in neonates and very small infants.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
The drug product is packaged in a clear plastic hypodermic syringe, which consists of a hypodermic barrel with luer lock, plunger stopper, plunger rod, and tip cap. There are two syringe sizes (6 mL and 12 mL), intended for single use and subsequent disposal. Medefil is approved for the following fill volumes
- 6 mL syringe: 1 mL, 2 mL, 2.5 mL, 3 mL, and 5 mL.
- 12 mL syringe: 3 mL, 5 mL, and 10 mL.
Currently, the drug product is marketed only as 10mL fill in 12mL syringe. The filled 6 mL and 12 mL syringes are packaged individually in plastic pouches.
DOSAGE FORM
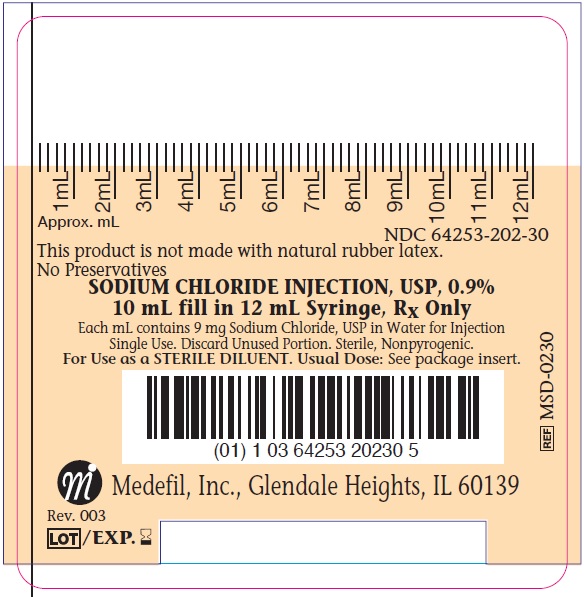
Product Number NDC Fill Volume MSD-0230 64253-202-30 10 mL fill in 12 mL syringe The above product is available in boxes of 30, 60 or 120 count each.
STORAGE AND HANDLING
Store at 25°C (77°F); excursions permitted to 15°-30°C (59° - 86°F). Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
17.1 When using to dilute drug products, consult the drug product manufacturer’s instruction to confirm compatibility, appropriate dilution or volume for dissolving drugs including the route and rate of injection. Inspect reconstituted (diluted or dissolved) drugs for clarity (if soluble) and freedom from unexpected precipitation or discoloration prior to administration. Do not use Sodium Chloride Injection, USP 0.9% if the solution is discolored, cloudy, hazy, or contains a precipitate, or if the syringe damaged [ see Warning and Precautions, section 5.1].
17.2 Syringes are for single use only. Discard unused portions and dispose of the unit in an appropriate sharps container. [ see Warning and Precautions, section 5.2].
Medefil, Inc., 250 Windy Point Drive, Glendale Heights, IL 60139. Prepared 03/2015 Rev. 002
Note: For detailed handling information see instruction for use.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64253-202 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 9 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64253-202-30 60 in 1 BOX 01/13/2012 1 10 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202832 01/13/2012 Labeler - Medefil, Inc. (016448669)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.