PHEBURANE- sodium phenylbutyrate pellet PHEBURANE- sodium phenylbutyrate kit
PHEBURANE by
Drug Labeling and Warnings
PHEBURANE by is a Prescription medication manufactured, distributed, or labeled by Medunik USA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PHEBURANE safely and effectively. See full prescribing information for PHEBURANE.
PHEBURANE® (sodium phenylbutyrate) oral pellets
Initial U.S. Approval: 1996INDICATIONS AND USAGE
PHEBURANE is a nitrogen-binding agent indicated as adjunctive therapy to standard of care, which includes dietary management, for the chronic management of adult and pediatric patients with urea cycle disorders (UCDs), involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinic acid synthetase (AS). (1)
Limitations of Use
PHEBURANE is not indicated for the treatment of acute hyperammonemia. (1)DOSAGE AND ADMINISTRATION
- PHEBURANE treatment should be supervised by a healthcare provider experienced in the treatment of UCDs. For administration, see full prescribing information. (2.1, 2.4)
- The recommended dosage measured as sodium phenylbutyrate is:
- Monitor plasma ammonia levels to determine the need for dosage adjustment. (2.2)
- Monitor patients for potential neurotoxicity. (2.2)
- For patients with hepatic impairment, start at the lower end of the recommended dosing range. (2.3)
DOSAGE FORMS AND STRENGTHS
Oral pellets: 84 g of sodium phenylbutyrate per bottle. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Neurotoxicity of Phenylacetate: Increased exposure to phenylacetate, the major metabolite of PHEBURANE, may be associated with neurotoxicity in patients with UCDs. Consider reducing the dose if neurotoxicity symptoms are present. (5.1)
- Hypokalemia: Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary. (5.2)
- Conditions Associated with Edema: Calculate the total amount of sodium patients will be exposed to based on their weight or body surface area. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of PHEBURANE and initiate appropriate therapy. (5.3)
- Diabetes Mellitus, Hereditary Fructose Intolerance, Glucose-Galactose Malabsorption or Sucrase-Isomaltase Insufficiency: Avoid use of PHEBURANE in patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 3%) are menstrual dysfunction, decreased appetite, body odor and bad taste or taste aversion. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Medunik USA, Inc. at 1-844-884-5520 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Adjustment and Monitoring
2.3 Dosage Adjustment in Patients with Hepatic Impairment
2.4 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neurotoxicity of Phenylacetate
5.2 Hypokalemia
5.3 Conditions Associated with Edema
5.4 Diabetes Mellitus, Hereditary Fructose Intolerance, Glucose-Galactose Malabsorption or Sucrase-Isomaltase Insufficiency
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Ammonia
7.2 Potential for Other Drugs to Affect PHEBURANE
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
PHEBURANE is indicated as adjunctive therapy to standard of care, which includes dietary management, for the chronic management of adult and pediatric patients with urea cycle disorders (UCDs), involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinic acid synthetase (AS).
Limitations of Use
Episodes of acute hyperammonemia may occur in patients while on PHEBURANE. PHEBURANE is not indicated for the treatment of acute hyperammonemia, which can be a life-threatening medical emergency that requires rapid acting interventions to reduce plasma ammonia levels.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
PHEBURANE treatment should be supervised by a healthcare provider experienced in the treatment of urea cycle disorders.
The recommended dosage of PHEBURANE (measured as sodium phenylbutyrate) for patients with urea cycle disorders is:
- Patients weighing less than 20kg: 450 – 600 mg/kg/day of sodium phenylbutyrate orally. Divide the calculated total daily dose into three to six doses. Administer as three to six divided doses and take with food.
- Patients weighing greater than or equal to 20 kg: 9.9 – 13 g/m2/day of sodium phenylbutyrate orally. Divide the calculated total daily dose into three to six doses. Administer as three to six divided doses and take with food.
The maximum dosage is 20 grams per day. Combine PHEBURANE with dietary protein restriction and, in some cases, amino acid supplementation (e.g., essential amino acids, arginine, citrulline, and protein-free calorie supplements).
Measure the dose using only the calibrated spoon provided in the packaging. This calibrated dosing spoon directly measures PHEBURANE oral pellets as sodium phenylbutyrate [see Dosage and Administration (2.4)].
If a dose is missed, take the missed dose as soon as possible. There should be at least 3 hours between two doses and doses should not be doubled to make up for the missed dose.
2.2 Dosage Adjustment and Monitoring
Monitor plasma ammonia levels to determine the need for dosage adjustment. Adjust the PHEBURANE dosage to maintain the plasma ammonia level within the normal range for the patient’s age, taking into consideration their clinical condition (e.g., nutritional requirements, protein intake, growth parameters, etc.).
Monitor patients for potential neurotoxicity and obtain measurements of plasma phenylacetate and phenylacetylglutamine levels [see Warnings and Precautions (5.1), Adverse Reactions (6)]. If neurologic symptoms (e.g. vomiting, nausea, headache, somnolence or confusion) are present in the absence of high ammonia levels or other intercurrent illnesses, consider reducing the dose of PHEBURANE.
2.3 Dosage Adjustment in Patients with Hepatic Impairment
For patients with hepatic impairment, start at the lower end of the recommended dosing range and maintain patients on the lowest dose necessary to control plasma ammonia levels [see Use in Specific Populations (8.7)].
2.4 Administration Instructions
For oral administration only. Administration via gastrostomy or nasogastric tubes has not been evaluated.
- Schedule PHEBURANE dosages at the same time as food consumption (meal or snack).
- Use the calibrated dosing spoon to measure PHEBURANE oral pellets. The dosing spoon is directly calibrated in grams of sodium phenylbutyrate.
- Swallow the coated oral pellets with a drink (e.g., water, fruit juices, protein-free infant formulas) or sprinkle onto spoonful of apple sauce or carrot puree. Do not chew PHEBURANE oral pellets directly or mix into liquids.
- Swallow immediately to minimize dissolution of coating.
Administration of PHEBURANE oral pellets with other foods has not been studied and is not recommended. Additionally, administration with soft food is only recommended in patients old enough to consume soft foods.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Neurotoxicity of Phenylacetate
Increased exposure to phenylacetate, the major metabolite of PHEBURANE, may be associated with neurotoxicity in patients with UCDs. In a study of adult cancer patients receiving intravenous phenylacetate, 250-300 mg/kg/day for 14 days, repeated at 4-week intervals, signs and symptoms of neurotoxicity, which were reversible upon discontinuation, were seen at plasma concentrations ≥ 3.5 mmol/L, and included somnolence, fatigue, and light headedness [see Adverse Reactions (6)]. PHEBURANE is not approved for intravenous use or for treatment of patients with cancer.
If symptoms of vomiting, nausea, headache, somnolence or confusion are present in the absence of high ammonia levels or other intercurrent illnesses, consider reducing the dose of PHEBURANE [see Dosage and Administration (2.2)].
Phenylacetate caused neurotoxicity when given subcutaneously in rat pups [see Use in Specific Populations (8.4)].
5.2 Hypokalemia
Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary.
5.3 Conditions Associated with Edema
PHEBURANE contains 124 mg (5.4 mmol) of sodium per gram of sodium phenylbutyrate, corresponding to 2.5 g (108 mmol) of sodium in the maximum daily dose of 20 g of sodium phenylbutyrate. In order to decide if administration of PHEBURANE is appropriate in patients with diseases that involve edema such as heart failure, cirrhosis, or nephrosis, calculate the total amount of sodium patients will be exposed to based on their weight or body surface area (BSA) [see Dosage and Administration (2.1)]. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of PHEBURANE and initiate appropriate therapy.
5.4 Diabetes Mellitus, Hereditary Fructose Intolerance, Glucose-Galactose Malabsorption or Sucrase-Isomaltase Insufficiency
PHEBURANE contains 768 mg of sucrose per gram of sodium phenylbutyrate, corresponding to 15.4 g of sucrose in the maximum daily dose of 20 g of sodium phenylbutyrate. This should be considered in patients with diabetes mellitus. Avoid use of PHEBURANE in patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency.
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of sodium phenylbutyrate were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily and from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Most common adverse reactions (incidence ≥ 3%) are amenorrhea or menstrual dysfunction (irregular menstrual cycles), decreased appetite, body odor and bad taste or taste aversion.
Less Common Clinical Adverse Reactions
Blood and lymphatic system disorders: aplastic anemia, ecchymoses
Cardiac disorders: arrhythmia
Gastrointestinal disorders: abdominal pain, decreased appetite, gastritis, nausea and vomiting, constipation, rectal bleeding, peptic ulcer disease, pancreatitis
Metabolism and nutrition disorders: increased weight, edema
Nervous system disorders: syncope, headache
Psychiatric disorders: depression
Renal and urinary disorders: renal tubular acidosis
Skin and subcutaneous tissue disorders: rash
Laboratory Adverse Reactions
Blood and lymphatic system disorders: anemia, leukopenia and leukocytosis, thrombocytopenia, thrombocytosis
Hepatobiliary disorders: hyperbilirubinemia, increased blood alkaline phosphatase, increased transaminases
Metabolism and nutrition disorders: acidosis, alkalosis, hyperchloraemia, hypophosphataemia, hyperuricemia, hyperphosphatemia, hypernatremia, hypokalemia, hypoalbuminemia, decreased total protein
Clinical Adverse Reactions with Use of Phenylacetate
Nervous system disorders: Neurotoxicity was reported in cancer patients receiving intravenous phenylacetate, the major metabolite of PHEBURANE (PHEBURANE is not approved for intravenous use or for treatment of patients with cancer). Signs and symptoms were predominately somnolence, fatigue, and dizziness (lightheadedness); less frequently reported were headache, dysgeusia, hypoacusis, disorientation, memory impairment, and exacerbation of a pre-existing neuropathy.
-
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Ammonia
Corticosteroids
Use of corticosteroids may cause the breakdown of body protein and increase plasma ammonia levels.
Valproic Acid and Haloperidol
Hyperammonemia may be induced by haloperidol and by valproic acid.
Monitor plasma ammonia levels closely when corticosteroids, valproic acid, or haloperidol is used concomitantly with PHEBURANE.
7.2 Potential for Other Drugs to Affect PHEBURANE
Probenecid
Probenecid may inhibit renal excretion of the metabolites of PHEBURANE including phenylacetate and phenylacetylglutamine. Monitor patients for potential neurotoxicity and measure plasma phenylacetate and phenylacetylglutamine levels when probenecid is used concomitantly with PHEBURANE [see Dosage and Administration (2.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with sodium phenylbutyrate use in pregnant women are insufficient to identify a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with sodium phenylbutyrate. Based on published animal data, phenylacetate may be neurotoxic to the developing brain (see Data).
There are serious risks to the mother and fetus associated with untreated urea cycle disorders during pregnancy which can result in serious morbidity and mortality to the mother and fetus (see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnancy is a time of increased metabolic demand which increases the risk for hyperammonemic episodes when metabolic demands are not met. Hyperammonemic episodes in pregnancy are associated with impaired cognition in the mother and an increased risk of maternal and fetal death.
In rats, intrauterine exposure to phenylacetate produced lesions in the neonatal brain in layer 5 of the cortical pyramidal cells; dendritic spines were longer and thinner than normal and reduced in number.
8.2 Lactation
Risk Summary
There are no data on the presence of sodium phenylbutyrate and its metabolite in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PHEBURANE and any potential adverse effects on the breastfed infant from PHEBURANE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of PHEBURANE have been established as adjunctive therapy to the standard of care, which includes dietary management, in the chronic management of pediatric patients with urea cycle disorders (UCDs), involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinic acid synthetase (AS).
PHEBURANE is not indicated for the treatment of acute hyperammonemia, which can be a life-threatening medical emergency that requires rapid acting interventions to reduce plasma ammonia levels.
The sodium content of PHEBURANE has the potential to cause new-onset edema or worsening edema from salt and water retention, particularly in patients with underlying predisposing conditions [see Warnings and Precautions (5.3)].
Neurotoxicity has been observed in juvenile animals with phenylacetate exposure [see Warnings and Precautions (5.1)].
Juvenile Animal Toxicity Data
When given subcutaneously to rat pups, 190–474 mg/kg phenylacetate caused decreased proliferation and increased loss of neurons, and it reduced CNS myelin. Cerebral synapse maturation was retarded, and the number of functioning nerve terminals in the cerebrum was reduced, which resulted in impaired brain growth.
8.5 Geriatric Use
Clinical studies of PHEBURANE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Renal Impairment
No studies with PHEBURANE were conducted in subjects with renal impairment. Monitor plasma ammonia levels when starting patients with impaired renal function on PHEBURANE [see Clinical Pharmacology (12)].
8.7 Hepatic Impairment
No studies with PHEBURANE were conducted in subjects with hepatic impairment. Start at the lower end of the recommended dosing range and maintain patients with hepatic impairment on the lowest dose necessary to control plasma ammonia levels [see Clinical Pharmacology (12.3), Dosage and Administration (2.3)].
-
10 OVERDOSAGE
Overdoses of PHEBURANE exceeding ten-fold the maximum recommended dosage may produce emesis, CNS depression, metabolic acidosis with or without respiratory alkalosis, hypernatremia, hypokalemia, and hypophosphatemia. Symptoms of overdose overlap with those of acute hyperammonemia. If overdose occurs, discontinue PHEBURANE, monitor plasma phenylacetate and ammonia levels closely, and institute appropriate emergency management, which may include hemodialysis, continuous veno-venous hemofiltration (CVVH) or extracorporeal membrane oxygenation (ECMO).
-
11 DESCRIPTION
PHEBURANE (sodium phenylbutyrate) oral pellets is a nitrogen binding agent. Sodium phenylbutyrate is a white or yellowish-white powder, freely soluble in water and in methanol, and practically insoluble in methylene chloride. It is known chemically as sodium 4-phenylbutanoate with a molecular weight of 186.19 and the molecular formula C10H11NaO2.
Structural formula:
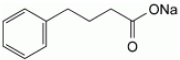
Each bottle of PHEBURANE contains 84 g of sodium phenylbutyrate (equivalent to 74 g of phenylbutyrate) in 174 g of oral pellets. Each gram of pellets contains 483 mg of sodium phenylbutyrate (equivalent to 423 mg of phenylbutyrate). PHEBURANE contains the following inactive ingredients: ethylcellulose, hypromellose, polyethylene glycol 1500, povidone K25, and sugar spheres.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sodium phenylbutyrate is a pro-drug and is metabolized to phenylacetate. Phenylacetate is a metabolically-active compound that conjugates with glutamine via acetylation to form phenylacetylglutamine. Phenylacetylglutamine is excreted by the kidneys, hence providing an alternate vehicle for waste nitrogen excretion.
12.2 Pharmacodynamics
In patients with urea cycle disorders, sodium phenylbutyrate decreased elevated plasma ammonia and glutamine levels.
12.3 Pharmacokinetics
The pharmacokinetics of phenylbutyrate and its metabolite phenylacetate were characterized in healthy adult subjects following a single oral administration of PHEBURANE (3 g of sodium phenylbutyrate) under fasted and fed conditions.
Absorption
Under fasted condition, the mean (SD) maximum plasma concentration (Cmax) of phenylbutyrate ranged from 146 (30) to 169 (33) μg/mL, which was achieved at approximately 0.6 hour after PHEBURANE administration. The mean (SD) area under the plasma concentration-time curve (AUC) of phenylbutyrate ranged from 272 (78) to 283 (68) h*μg/mL.
Effect of Food
Compared to fasted condition, AUC of phenylbutyrate decreased by 40-45% and Cmax of phenylbutyrate decreased by 55%, when PHEBURANE was administered with a high-fat, high-calorie meal (total 800 to 1000 calories with approximately 150, 250, and 500-600 calories from protein, carbohydrate, and fat, respectively). When PHEBURANE was administered with a normal-fat, normal-calorie, low-protein meal (total 600 to 700 calories with approximately 60, 200, and 400 calories from protein, fat, and carbohydrate, respectively), AUC of phenylbutyrate decreased by 56-63% and Cmax decreased by 43-45% compared to fasted condition.
Elimination
The mean half-life of phenylbutyrate ranged from 0.5 to 0.8 hour.
Metabolism
Following oral administration, sodium phenylbutyrate is metabolized by β-oxidation into phenylacetate, which is converted to its coenzyme A ester, phenylacetyl-coenzyme A and further conjugated with glutamine to form phenylacetylglutamine. Phenylacetylglutamine is excreted by the kidneys. The major sites for metabolism of sodium phenylbutyrate are the liver and kidneys. Phenylacetate is also hydrolysed by esterases in liver and blood.
Excretion
Approximately 80–100% of sodium phenylbutyrate is excreted by the kidneys within 24 hours as phenylacetylglutamine. For each gram of sodium phenylbutyrate administered, it is estimated that between 0.12–0.15 grams of phenylacetylglutamine nitrogen are produced.
Specific Populations
Patients with Renal Impairment or Hepatic Impairment
PHEBURANE has not been studied in patients with renal impairment or in patients with hepatic impairment.
Drug Interaction Studies
In vitro or clinical studies with PHEBURANE for determination of potential drug-drug interaction have not been conducted.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PHEBURANE (sodium phenylbutyrate) oral pellets consists of white to off-white pellets and is available in a child-resistant high-density polyethylene (HDPE) bottle with a desiccant in the cap.
Each bottle contains 84 g of sodium phenylbutyrate (equivalent to 74 g of phenylbutyrate) in 174 g of oral pellets (NDC: 71770-200-10). Each gram of pellets contains 483 mg of sodium phenylbutyrate (equivalent to 423 mg of phenylbutyrate).
A calibrated measuring spoon that dispenses up to 3 g of sodium phenylbutyrate in increments of 0.25 g is provided in the packaging.
Store PHEBURANE at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Discard any remaining PHEBURANE 45 days after first opening of the bottle.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Neurotoxicity
Advise the patient or caregiver that neurotoxicity may occur during PHEBURANE treatment. Inform the patient or caregiver of the signs and symptoms of this risk and to contact the healthcare provider immediately if signs and symptoms occur [see Warnings and Precautions (5.1)].
Administration
Instruct the patient or caregiver to only use the calibrated dosing spoon supplied with PHEBURANE to measure the oral pellets. Inform the patient or caregiver that the dosing spoon is directly calibrated in grams of sodium phenylbutyrate. Inform the patient or caregiver to consume the pellets immediately after preparation [see Dosage and Administration (2.4)].
Inform the patient or caregiver that if a dose is missed, take the missed dose as soon as possible. Instruct the patient or caregiver that there should be at least 3 hours between two doses and doses should not be doubled to make up for the missed dose [see Dosage and Administration (2.1)].
Storage and Handling
Advise the patient or caregiver to discard any remaining PHEBURANE 45 days after first opening of the bottle.
Distributed by:
Medunik USA, Inc.
2 Research Way, Suite 1B
Princeton, NJ 08540
PHEBURANE® is a registered trademark of Lucane Pharma
-
PATIENT PACKAGE INSERT
Patient Information
PHEBURANE (FE bue rayne)
(sodium phenylbutyrate)
oral pellets
What is PHEBURANE?
- PHEBURANE is a prescription medicine, used along with a specific diet, for the long-term management of adults and children with urea cycle disorders (UCDs), involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinate synthetase (AS).
- Episodes of sudden, rapid increase of ammonia in the blood (acute hyperammonemia) may happen in people during treatment with PHEBURANE. PHEBURANE is not used for the treatment of acute hyperammonemia, which can be life-threatening and requires emergency medical treatment.
Before taking PHEBURANE, tell your or your child’s healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have kidney or liver problems.
- have diabetes (PHEBURANE contains sucrose), or have a history of fructose intolerance, glucose-galactose malabsorption, or sucrase-isomaltase insufficiency.
- are pregnant or plan to become pregnant. It is not known if PHEBURANE will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if PHEBURANE passes into your breastmilk. Talk to your healthcare provider about the best way to feed your baby during treatment with PHEBURANE.
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Certain medicines may increase the level of ammonia in your blood or cause serious side effects when taken during treatment with PHEBURANE. Especially tell your healthcare provider if you or your child take:
- corticosteroids
- valproic acid
- haloperidol
- probenecid
Know the medicines you take. Keep a list of them to show your or your child’s healthcare provider and pharmacist when you get a new medicine.
How should I take PHEBURANE?
Read the detailed Instructions for Use that comes with PHEBURANE for information about the right way to prepare and take a dose of PHEBURANE.
- Take PHEBURANE exactly as prescribed by your healthcare provider.
- Your healthcare provider may change your dose if needed. Do not change your dose unless your healthcare provider tells you to.
- Your healthcare provider will prescribe PHEBURANE based on your or your child’s weight.
- Schedule your PHEBURANE dose at the same time you eat a meal or snack.
- Use the calibrated dosing spoon to measure PHEBURANE oral pellets.
- PHEBURANE oral pellets should be taken with a drink such as water, fruit juices or protein-free infant formulas, orsoft food, such as apple sauce or carrot puree. It is not recommended to use other foods to take your dose of PHEBURANE oral pellets.
- Do not chew PHEBURANE oral pellets directly or mix PHEBURANE oral pellets into liquids.
- If you miss a dose of PHEBURANE, take it as soon as possible. Each dose of PHEBURANE should be taken at least 3 hours apart. If you have missed a dose within 3 hours of the next dose, take only one dose the next time it is scheduled.
- Do not give or take PHEBURANE through a gastrotomy or nasogastric tube.
- If you take too much PHEBURANE, call your healthcare provider or Poison Help line at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What are the possible side effects of PHEBURANE?
PHEBURANE can cause serious side effects, including:
Nervous system problems (neurotoxicity). Call your healthcare provider right away if get any of the following symptoms during treatment with PHEBURANE:
- sleepiness
- tiredness
- lightheadedness
- vomiting
- nausea
- headache
- confusion
Low potassium levels in your blood (hypokalemia). Your healthcare provider will monitor your blood potassium levels during treatment with PHEBURANE and treat if needed.
Conditions related to swelling (edema). PHEBURANE contains salt (sodium), which can cause swelling from salt and water retention. Your healthcare provider will decide if PHEBURANE is right for you if you have certain medical conditions that can cause swelling, such as heart failure, liver problems or kidney problems.
The most common side effects of PHEBURANE include:
- absent or irregular menstrual periods
- decreased appetite
- body odor
- bad taste or avoiding foods that you ate prior to getting sick (taste aversion)
Your healthcare provider may do certain blood tests to check you or your child for side effects during treatment with PHEBURANE.
These are not all of the possible side effects of PHEBURANE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PHEBURANE?
- Store PHEBURANE at room temperature between 68°F and 77°F (20°C and 25°C).
- PHEBURANE comes in a child-resistant bottle. The bottle cap contains a desiccant to help keep PHEBURANE dry.
- Throw away (discard) any remaining PHEBURANE 45 days after first opening of the bottle.
Keep PHEBURANE and all medicines out of the reach of children.
General information about the safe and effective use of PHEBURANE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PHEBURANE for a condition for which it was not prescribed. Do not give PHEBURANE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about PHEBURANE that is written for health professionals.
What are the ingredients in PHEBURANE?
Active ingredient: sodium phenylbutyrate
Inactive Ingredients: ethylcellulose, hypromellose, polyethylene glycol 1500, povidone K25, and sugar spheres.
Distributed by: Medunik USA, Inc., 2 Research Way, Suite 1B, Princeton, NJ 08540
For more information, go to www.medunikusa.com
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: August 2023
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
PHEBURANE ®(FE bue rayne)
(sodium phenylbutyrate)
oral pelletsRead this Instructions for Use before taking PHEBURANE oral pellets and each time you get a refill. There may be new information. This Instructions for Use does not take the place of talking to your healthcare provider about your medical condition or treatment. Talk to your healthcare provider or pharmacist if you have any questions about how to take a dose of PHEBURANE.
This Instructions for Use contains information on how to measure and take PHEBURANE.
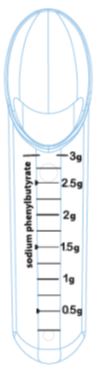
Figure A: Calibrated dosing spoon provided
with PHEBURANEImportant information you need to know before taking PHEBURANE
- The total daily dose of PHEBURANE must be given in divided doses and taken with food (meal or snack). Your healthcare provider will tell you when to take PHEBURANE and the dose to take.
- Schedule PHEBURANE doses at the same time as eating a meal or snack.
- Only use the calibrated dosing spoon provided with PHEBURANE to measure the dose (See Figure A).
Supplies needed for measuring and taking PHEBURANE
- Bottle of PHEBURANE oral pellets.
- Calibrated dosing spoon provided with PHEBURANE.
- A drink such as water, fruit juices or protein-free infant formulas, orsoft food, such as apple sauce or carrot puree. It is not recommended to use other foods to take your dose of PHEBURANE oral pellets.
Measuring PHEBURANE
Repeat Steps 1 through 3 throughout the day as prescribed by your healthcare provider to get the total daily dose.
Step 1
- Use the calibrated dosing spoon to measure the prescribed divided dose of PHEBURANE oral pellets (See Figure A).
The calibrated dosing spoon measures PHEBURANE oral pellets as grams (g) of sodium phenylbutyrate. The calibrated dosing spoon dispenses up to 3 g of sodium phenylbutyrate, in increments of 0.25 g.
- Pour the oral pellets directly into the calibrated dosing spoon until it reaches the black line for the prescribed dose of sodium phenylbutyrate in grams (See Figure B).
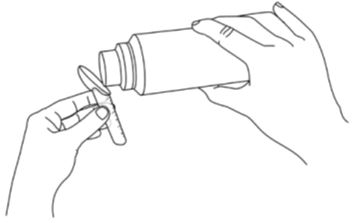
Figure B: Pour the PHEBURANE oral
pellets directly into the calibrated
dosing spoon.Taking PHEBURANE
Step 2
- Tap the bottom of the calibrated dosing spoon 1 time on a hard surface to give a flat level of oral pellets and continue filling until you reach the prescribed dose.
Step 3
- Swallow PHEBURANE oral pellets with a drink such as water, fruit juices, or protein-free infant formulas.
or
- Sprinkle PHEBURANE oral pellets onto a spoonful of apple sauce or carrot puree and swallow right away. It is important to swallow right away to prevent coating from dissolving.
Do not chew PHEBURANE oral pellets directly or mix PHEBURANE oral pellets into liquids.
If the prescribed divided dose is more than 3 grams, repeat Steps 1 through 3 to get the prescribed divided dose of sodium phenylbutyrate.
Storing PHEBURANE
- Store PHEBURANE at room temperature between 68°F and 77°F (20°C and 25°C).
- PHEBURANE comes in a child-resistant bottle. The bottle cap contains a desiccant to help keep PHEBURANE dry.
- Throw away (discard) any remaining PHEBURANE 45 days after first opening of the bottle.
- Keep PHEBURANE and all medicines out of the reach of children.
Disposing of PHEBURANE
- Throw away (dispose of) the bottle of PHEBURANE 45 days after first opening by dropping off the medicine at a drug take back site or following the steps below.
- Mix medicine with an unappealing substance such as dirt, cat litter, or used coffee grounds
- Place the mixture in a container such as a sealed plastic bag
- Throw away the container in your trash at home, and
- Delete all personal information on the prescription label of the empty medicine bottle, then throw away or recycle the empty bottle.
- Throw away (dispose of) used calibrated dosing spoon in the household trash.
- Do not use the calibrated dosing spoon to measure any other medicine.
If cleaning is necessary, rinse the calibrated dosing spoon with water and dry it completely.
To replace the calibrated dosing spoon, contact Medunik USA at 1-844-884-5520.
Distributed by: Medunik USA, Inc. 2 Research Way, Suite 1B, Princeton, NJ 08540

PHEBURANE® is a registered trademark of Lucane Pharma.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: August 2023
-
PRINCIPAL DISPLAY PANEL - Carton
NDC: 71770-200-10
Pheburane®
(sodium phenylbutyrate)
oral pellets84 g per bottle*
Rx only
Pharmacist: Dispense the enclosed
instructions per use with each prescriptionUse only the enclosed measuring spoon
designed for use with this product.Medunik USA
-
PRINCIPAL DISPLAY PANEL - Container
NDC: 71770-210-10
Pheburane®
(sodium phenylbutyrate)
oral pellets84 g per bottle*
For oral use only
Rx onlyUse only the enclosed
measuring spoon designed for
use with this product.
-
INGREDIENTS AND APPEARANCE
PHEBURANE
sodium phenylbutyrate pelletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71770-210 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHENYLBUTYRATE (UNII: NT6K61736T) (PHENYLBUTYRIC ACID - UNII:7WY7YBI87E) SODIUM PHENYLBUTYRATE 483 mg in 1 g Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE K25 (UNII: K0KQV10C35) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71770-210-10 174 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 09/14/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216513 09/14/2022 PHEBURANE
sodium phenylbutyrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71770-200 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71770-200-10 1 in 1 CARTON 08/26/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 174 g Part 1 of 1 PHEBURANE
sodium phenylbutyrate pelletProduct Information Item Code (Source) NDC: 71770-210 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHENYLBUTYRATE (UNII: NT6K61736T) (PHENYLBUTYRIC ACID - UNII:7WY7YBI87E) SODIUM PHENYLBUTYRATE 483 mg in 1 g Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE K25 (UNII: K0KQV10C35) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71770-210-10 174 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216513 09/14/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216513 08/26/2022 Labeler - Medunik USA (080318531)
Trademark Results [PHEBURANE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PHEBURANE 79117600 4281315 Live/Registered |
LUCANE PHARMA 2012-06-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.