ICY COOL MAXIMUM STRENGTH- menthol gel
Icy Cool by
Drug Labeling and Warnings
Icy Cool by is a Otc medication manufactured, distributed, or labeled by C.D.M.A. Inc., NATURAL ESSENTIALS, INC., NATURAL ESSENTIALS INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- a transient burning sensation may occur upon application but generally disappears in several days.
- Directions
- Inactive ingredients –
-
Principal Display Panel - 3 oz. Roll on Label
QC Quality Choice
NDC: 63868-632-82
*Compare to BIOFREEZE®
Pain Relieving GelMaximum Strength
Icy CoolTM with aloe
Pain Relieving Roll-On
Natural Menthol 5.5% | Topical Analgesic
Relief From Aches and Pains Associated with Strains, Sprains, Backaches, Bruises and Arthritis
1 Roll-on 3oz./89mL.
-
INGREDIENTS AND APPEARANCE
ICY COOL MAXIMUM STRENGTH
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63868-632 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol 55 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL ALCOHOL (UNII: ND2M416302) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63868-632-82 89 mL in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2013 Labeler - C.D.M.A. Inc. (011920774) Registrant - NATURAL ESSENTIALS, INC. (947484713) Establishment Name Address ID/FEI Business Operations NATURAL ESSENTIALS INC. 947484713 MANUFACTURE(63868-632)
Trademark Results [Icy Cool]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
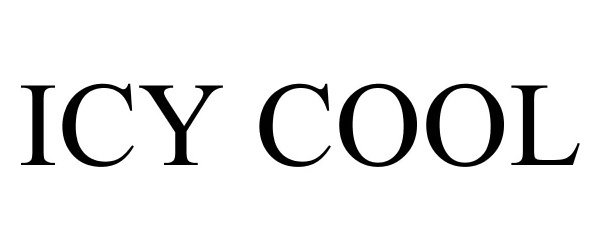 ICY COOL 87563901 5681460 Live/Registered |
MerchSource, LLC 2017-08-10 |
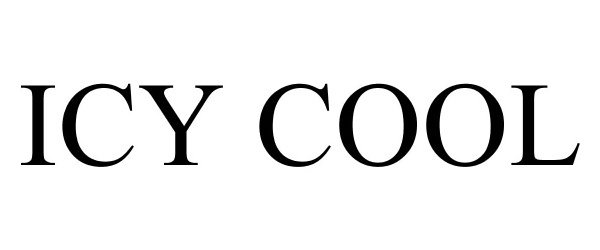 ICY COOL 85429575 not registered Dead/Abandoned |
Guthy-Renker LLC 2011-09-22 |
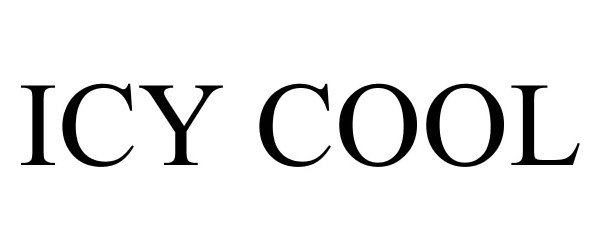 ICY COOL 78855645 not registered Dead/Abandoned |
Johnson & Johnson 2006-04-06 |
 ICY COOL 74196128 not registered Dead/Abandoned |
Procter & Gamble Company, The 1991-08-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
