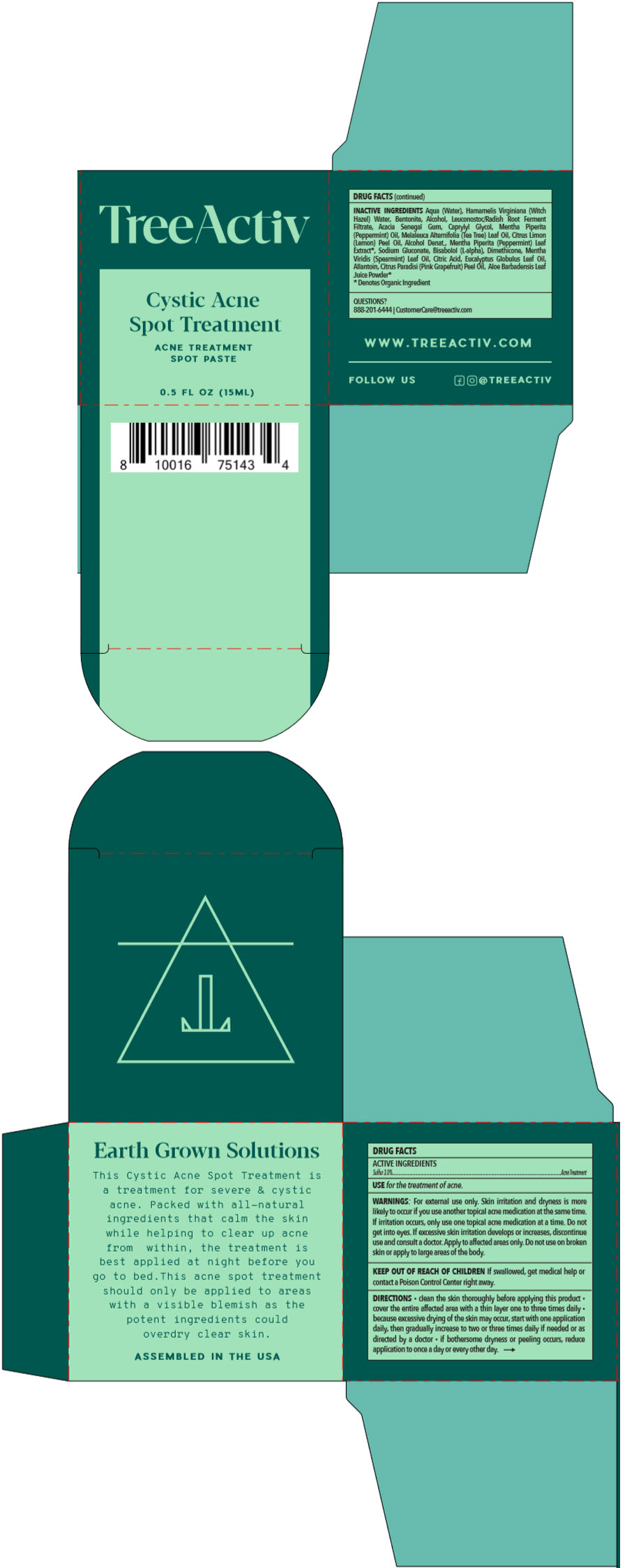
TREEACTIV CYSTIC ACNE SPOT TREATMENT- sulfur paste
TreeActiv Cystic Acne Spot Treatment by
Drug Labeling and Warnings
TreeActiv Cystic Acne Spot Treatment by is a Otc medication manufactured, distributed, or labeled by Leiluna LLC, Island Kinetics, Inc. d.b.a. CoValence Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENTS
- PURPOSE
- USE
-
WARNINGS
For external use only. Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time. Do not get into eyes. If excessive skin irritation develops or increases, discontinue use and consult a doctor. Apply to affected areas only. Do not use on broken skin or apply to large areas of the body.
-
DIRECTIONS
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INACTIVE INGREDIENTS
Aqua (Water), Hamamelis Virginiana (Witch Hazel) Water, Bentonite, Alcohol, Leuconostoc/Radish Root Ferment Filtrate, Acacia Senegal Gum, Caprylyl Glycol, Mentha Piperita (Peppermint) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Citrus Limon (Lemon) Peel Oil, Alcohol Denat., Mentha Piperita (Peppermint) Leaf Extract1, Sodium Gluconate, Bisabolol (L-alpha), Dimethicone, Mentha Viridis (Spearmint) Leaf Oil, Citric Acid, Eucalyptus Globulus Leaf Oil, Allantoin, Citrus Paradisi (Pink Grapefruit) Peel Oil, Aloe Barbadensis Leaf Juice Powder1
- 1 Denotes Organic Ingredient
- QUESTIONS?
- PRINCIPAL DISPLAY PANEL - 15 ML Jar Box
-
INGREDIENTS AND APPEARANCE
TREEACTIV CYSTIC ACNE SPOT TREATMENT
sulfur pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72841-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) BENTONITE (UNII: A3N5ZCN45C) ALCOHOL (UNII: 3K9958V90M) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) ACACIA (UNII: 5C5403N26O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PEPPERMINT OIL (UNII: AV092KU4JH) TEA TREE OIL (UNII: VIF565UC2G) LEMON OIL (UNII: I9GRO824LL) MENTHA PIPERITA LEAF (UNII: A389O33LX6) LEVOMENOL (UNII: 24WE03BX2T) SODIUM GLUCONATE (UNII: R6Q3791S76) DIMETHICONE (UNII: 92RU3N3Y1O) MENTHA SPICATA OIL (UNII: C3M81465G5) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EUCALYPTUS OIL (UNII: 2R04ONI662) ALLANTOIN (UNII: 344S277G0Z) GRAPEFRUIT OIL (UNII: YR377U58W9) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72841-201-01 1 in 1 BOX 05/07/2020 1 15 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 05/07/2020 Labeler - Leiluna LLC (080065214) Establishment Name Address ID/FEI Business Operations Island Kinetics, Inc. d.b.a. CoValence Laboratories 959735002 MANUFACTURE(72841-201)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.