CYTALUX- pafolacianine injection injection
Cytalux by
Drug Labeling and Warnings
Cytalux by is a Prescription medication manufactured, distributed, or labeled by On Target Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CYTALUX safely and effectively. See full prescribing information for CYTALUX.
CYTALUX® (pafolacianine) injection, for intravenous use
Initial U.S. Approval: 2021RECENT MAJOR CHANGES
Dosage and Administration, 7/2025
Preparation and Storage Instructions ( 2.3)
INDICATIONS AND USAGE
CYTALUX is an optical imaging agent indicated as an adjunct for intraoperative identification of:
- Malignant lesions in adult patients with ovarian cancer.
- Malignant and non-malignant pulmonary lesions in adult patients with known or suspected cancer in the lung. ( 1)
DOSAGE AND ADMINISTRATION
- For recommended testing, evaluations, and premedications, see Full Prescribing Information. ( 2.1)
- Recommended intravenous dosage of CYTALUX is:
- Adult Patients with Ovarian Cancer: 0.025 mg/kg over 60 minutes, 1 hour to 9 hours prior to surgery
- Adult Patients with Known or Suspected Cancer in the Lung: 0.025 mg/kg over 60 minutes, 1 hour to 24 hours prior to surgery. ( 2.2)
- For preparation, management of infusion-related reactions, and imaging information see Full Prescribing Information. CYTALUX should only be used by trained surgeons using FDA cleared imaging systems. ( 2.3, 2.4, 2.5)
DOSAGE FORMS AND STRENGTHS
Injection: 3.2 mg/1.6 mL (2 mg/mL) of pafolacianine in a single-dose
vial. (3)CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Infusion-Related Reactions: Interrupt the infusion and treat as
necessary with antihistamines and/or nausea medications. ( 5.1)
- Risk of misinterpretation: Non-fluorescing tissue in the surgical field
does not rule out the presence of tumor. Fluorescence may be seen
in non-cancerous tissues. ( 5.2)- Embryo-Fetal Toxicity: CYTALUX may cause fetal harm. Advise
females of reproductive potential of the potential risk to a fetus. ( 5.3,
8.1, 8.3)- Risk of Pafolacianine Aggregation and Infusion Reactions: Use only
5% Dextrose Injection for dilution. Do not use other diluents. ( 5.4)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥1%; ovarian and lung combined) included nausea, vomiting, abdominal pain, flushing, other infusion-related reactions, hypersensitivity, elevation in blood pressure, dyspepsia, and chest discomfort. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact On Target
Laboratories at 1-844-434-9333 or FDA at 1-800-FDA-1088 or
www.fda.gov/medwatch.DRUG INTERACTIONS
Folate Supplements: Avoid folate, folic acid, or folate-containing
supplements within 48 hours before administration of CYTALUX. (7)See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1. INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing, Evaluations and Premedications Prior to Administration of
CYTALUX2.2 Recommended Dosage and Administration
2.3 Preparation and Storage Instructions
2.4 Management of Infusion-Related Reactions
2.5 Imaging
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infusion-Related Reactions
5.2 Risk of Misinterpretation
5.3 Embryo-Fetal Toxicity
5.4 Risk of Pafolacianine Aggregation and Infusion Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Patients with Known or Suspected Ovarian Cancer
14.2 Patients with Known or Suspected Cancer in the Lung
16 HOW SUPPLIED/STORAGE AND HANDLING
10 Vial Carton
Vial Label
Single Vial Carton Label
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1. INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing, Evaluations and Premedications Prior to Administration of
CYTALUXObtain a pregnancy test in females of reproductive potential and verify the absence of pregnancy
prior to administration of CYTALUX [ see Warnings and Precautions (5.3) and Use in Specific
Populations ( 8.1, 8.3) ].
Discontinue folate, folic acid, or folate containing supplements 48 hours before administration of
CYTALUX [ see Drug Interactions (7)].
Consider administering antihistamines and/or anti-nausea medication for prophylaxis against infusion related
reactions [ see Warnings and Precautions (5.1)].2.2 Recommended Dosage and Administration
Adult Patients with Ovarian Cancer
The recommended dose of CYTALUX is a single intravenous infusion of 0.025 mg/kg diluted in 250 mL of 5% Dextrose Injection, administered over 60 minutes using a dedicated infusion line, 1 hour to 9 hours prior to surgery.
Adult Patients with Known or Suspected Cancer in the Lung
The recommended dose of CYTALUX is a single intravenous infusion of 0.025 mg/kg diluted in 250 mL of 5% Dextrose Injection, administered over 60 minutes using a dedicated infusion line, 1 hour to 24 hours prior to surgery.
2.3 Preparation and Storage Instructions
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
1. Use aseptic technique for the preparation of CYTALUX infusion solution.
2. Only use 5% Dextrose Injection for dilution. Do not use other diluents due to incompatibility [see Warnings and Precautions ( 5.4)] .
3. CYTALUX vials should be stored and thawed in the original carton protected from light.
- Remove carton containing one single vial of CYTALUX from freezer and record the date, time and thawing condition on the carton.
- Thaw at room temperature between 20°C to 25°C (68°F to 77°F) for at least 60 minutes or under refrigerated conditions between 2°C to 8°C (36°F to 46°F) for at least 6 hours. When thawed under refrigerated conditions, allow the vial to stand at room temperature for 15 minutes before dilution.
- Once thawed, an individual CYTALUX vial may be stored at room temperature between 20°C to 25°C (68°F to 77°F) for a maximum single period of 24 hours or under refrigerated conditions between 2°C to 8°C (36°F to 46°F) for a maximum single period of up to 30 days, prior to preparation for infusion.
- If CYTALUX vial is not used within the maximum single period at either room temperature or under refrigerated conditions, the vial may be refrozen up to three times.
- If the vial has been thawed after the third refreeze, it must be used. If not used after the third refreeze, do not use and discard the vial.
- Each vial of CYTALUX may be penetrated only once at the time of preparation of infusion solution. Once penetrated, the vial may not be refrozen.
4. Hand shake or vortex the thawed CYTALUX vial for 60 seconds.
5. Withdraw the calculated volume of CYTALUX for a dose of 0.025 mg/kg. Discard any unused portion in the vial.
6. Add into a 250 mL of 5% Dextrose Injection, USP bag.
7. Gently swirl the bag by hand for 1 minute to mix the solution.
8. Visually inspect the infusion bag. The solution should be light blue/green to clear in color and should not contain any visible particulate matter.
9. Protect the infusion bag from light using a light-blocking cover during infusion and storage.
10. If not immediately used, store the diluted CYTALUX infusion solution in a refrigerator at 2°C to 8°C (36°F to 46°F) for not more than 24 hours. Once the bag is removed from refrigeration, infusion must be completed within 3 hours.
2.4 Management of Infusion-Related Reactions
If the patient develops an infusion reaction during administration, interrupt the infusion and treat with
antihistamines and/or anti-nausea medication as necessary, based on clinical decision. Complete the
infusion within 3 hours of the start of the initial administration [ see Warnings and Precautions (5.1)].2.5 Imaging
- Clinical data demonstrate that near infrared (NIR) imaging devices that excite at 760 nm to 785 nm and detect emission at 790 nm to 815 nm are suitable for use with CYTALUX.
- CYTALUX is to be used with an NIR imaging system cleared by the FDA for specific use with pafolacianine.
- CYTALUX should only be used by surgeons who have completed a training program on the use of NIR imaging systems for fluorescence imaging during surgery. Training is provided by the device manufacturer.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Infusion-Related Reactions
Adverse reactions including nausea, vomiting, abdominal pain, flushing, hypersensitivity, elevation in blood pressure, dyspepsia, and chest discomfort were reported in patients receiving CYTALUX in clinical studies. A total of 17% of patients experienced reactions during the administration of CYTALUX [see Adverse Reactions ( 6.1)] . Reactions typically occurred within 15 minutes of the start of infusion. CYTALUX infusion interruption or discontinuation due to adverse reactions occurred in 11% of all patients. Treatment with antihistamines and/or anti-nausea medication may be used. If an adverse reaction occurs during administration, the infusion can be interrupted and resumed after treatment of the reaction [see Dosage and Administration ( 2.1, 2.4)] .
5.2 Risk of Misinterpretation
Errors may occur with the use of CYTALUX during intraoperative fluorescence imaging to detect ovarian cancer and lesions in the lung, including false negatives and false positives. Non-fluorescing tissue in the surgical field does not rule out the presence of ovarian cancer or lesions in the lung [see Clinical Studies (14)]. Fluorescence may be seen in normal tissues including bowel, kidneys, lymph nodes, and lungs as well as in inflamed tissues.
5.3 Embryo-Fetal Toxicity
Based on its mechanism of action, CYTALUX may cause fetal harm when administered to a pregnant
woman. Advise females of reproductive potential of the potential risk to a fetus. Verify pregnancy
status of females of reproductive potential prior to initiating CYTALUX treatment. [ see Use in Specific
Populations ( 8.1, 8.3), Clinical Pharmacology ( 12.1) ].5.4 Risk of Pafolacianine Aggregation and Infusion Reactions
Use of the incorrect diluent to prepare the CYTALUX infusion solution can cause the aggregation of
pafolacianine; aggregation may induce infusion reactions, such as nausea, vomiting, abdominal pain
or rash. Use only 5% Dextrose Injection to prepare the CYTALUX infusion solution. Do not use other
diluents. [see Dosage and Administration ( 2.3)]. -
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
Infusion-Related Reactions [ see Warnings and Precautions ( 5.1) ]6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CYTALUX was evaluated in four open label clinical studies, two studies (N = 44 and N = 150) in patients with ovarian cancer and two studies (N = 100 and N = 112) in patients with known or suspected cancer in the lung. A total of 406 patients received 0.025 mg/kg of CYTALUX via intravenous administration.
The demographic characteristics of the study population were 82% female (66% female in lung studies), mean age 64 years (range 26 to 89 years), 85% White, 6% Black or African American, 5% Asian, and 4% other race, 5% Hispanic or Latino, 92% Not Hispanic or Latino, and 3% unreported ethnicity.
Adverse reactions that occurred in > 1% of patients are presented in Table 1.
Table 1. Adverse Reactions from Clinical Studies Reported in ≥ 1% of CYTALUX Treated Patients with Ovarian Cancer or Known or Suspected Cancer in the Lung
Adverse Reaction CYTALUX 0.025 mg/kg
(N = 406)
%Nausea 13
Vomiting 5 Abdominal pain 2 Flushing 2
Other infusion-related reactions 2 Hypersensitivity 2 Elevation in blood pressure 1 Dyspepsia 1 Chest discomfort 1 Adverse reactions occurred during the administration of CYTALUX in 17% of patients.
Overall, the safety profile observed in patients treated with CYTALUX 0.025 mg/kg was similar between patients with ovarian cancer and patients with known or suspected cancer in the lung.
-
7 DRUG INTERACTIONS
Use of folate, folic acid, or folate-containing supplements may reduce binding of pafolacianine to
folate receptors and could reduce the detection of lesions with CYTALUX. Avoid administration of
folate, folic acid, or folate-containing supplements within 48 hours before administration of CYTALUX [see Dosage and Administration (2.1) and Clinical Pharmacology (12.1)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, pafolacianine may cause fetal harm when administered to a
pregnant woman [see Clinical Pharmacology (12.1)]. There are no available human data to evaluate
for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
No adverse developmental effects were observed in rats and rabbits with intravenous administration
of pafolacianine during organogenesis (embryofetal development) at doses up to 158-fold (rat) and 570-fold (rabbit) the recommended human dose of 0.025 mg/kg based on AUC, otherwise 9.6 and
38.4-fold based on human equivalent dose (HED) ( see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations
are unknown. All pregnancies have a background risk of birth defects, loss, or other adverse
outcomes. In the U.S. general population, the estimated background risk of major birth defects and
miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.Data
Animal Data
In the definitive embryo-fetal development (EFD) studies, pafolacianine was intravenously
administered during the period of organogenesis as follows: 0.015, 0.15, and 1.5 mg/kg/day from
gestational day (GD) 6 to GD17 in rats (HEDs of 0.002, 0.024 and 0.242 mg/kg/day), and 0.3, 1, and
3 mg/kg/day from GD7 to GD20 in rabbits (HEDs of 0.097, 0.323 and 0.968 mg/kg/day). No
significant drug-related maternal toxicity and embryo-fetal development toxicity were observed.
NOAELs were 1.5 mg/kg/day in rats and 3 mg/kg/day in rabbits. Estimated systemic exposures were
158 times (rat) and 570 times (rabbit) the human exposure at a human dose of 0.025 mg/kg based on
plasma AUC comparison.8.2 Lactation
Risk Summary
There are no data on the presence of pafolacianine in either human or animal milk, the effects on the
breastfed infant, or the effects on milk production. The developmental and health benefits of
breastfeeding should be considered along with the mother’s clinical need for CYTALUX and any
potential adverse effects on the breastfed infant from CYTALUX or from the underlying maternal
condition.8.3 Females and Males of Reproductive Potential
CYTALUX may cause fetal harm if administered to a pregnant woman [see Warnings And
Precautions ( 5.3) and Use in Specific Populations (8.1)].Pregnancy Testing
Obtain a pregnancy test in females of reproductive potential and verify the absence of
pregnancy prior to administration of CYTALUX [see Dosage and Administration (2.1)].8.4 Pediatric Use
Safety and effectiveness of CYTALUX in pediatric patients have not been established.8.5 Geriatric Use
Of the total number of patients in clinical studies of CYTALUX, 221 patients (54%) were 65 years of
age and older: 153 (38%) were 65 to 74 years of age, 66 (16%) were 75 to 84 years of age, and 2
(0.5%) were 85 years of age and older. No overall differences in safety, effectiveness, or
pharmacokinetics were observed between patients 65 years of age and older and younger adult
patients. -
11 DESCRIPTION
CYTALUX contains pafolacianine, an optical imaging agent, as a tetrasodium salt referred to as
pafolacianine sodium. Chemically, pafolacianine sodium is (S)-2-(4-(((2-amino-4-oxo-3,4-
dihydropteridin-6-yl)methyl)amino)benzamido)-3-(4-(((E)-2-((E)-2-(3,3-dimethyl-5-sulfonato-1-(4-
sulfonatobutyl)-3H-indol-1-ium-2-yl)vinyl)-6-((E)-2-(3,3-dimethyl-5-sulfonato-1-(4-
sulfonatobutyl)indolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)oxy)phenyl)propanoate hydrate
tetrasodium. Pafolacianine sodium has a molecular formula of C 61H 63N 9Na 4O 17S 4, a molecular mass
of 1414.42 g/mol and has the following structure: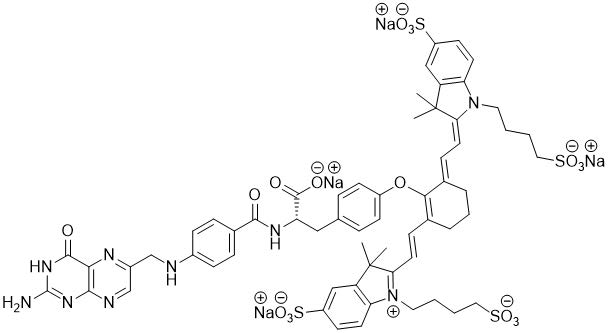
CYTALUX (pafolacianine) injection is a sterile, non-pyrogenic, dark bluish green, clear aqueous
solution for intravenous use. Each vial contains 3.2 mg (2 mg/mL) pafolacianine (equivalent to 3.4 mg
pafolacianine sodium),14.4 mg sodium chloride, 0.23 mg potassium phosphate monobasic, 1.27 mg
sodium phosphate dibasic heptahydrate in 1.6 mL volume. The pH is adjusted with sodium hydroxide
and/or hydrochloric acid and is between 7.1 to 7.8. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pafolacianine binds to alpha and beta folate receptors (FR) on cells, is internalized via receptor-mediated endocytosis, and accumulates intracellularly.
Pafolacianine enhances lesion visualization through the differential level of expression and accessibility of folate receptors within lesions relative to normal tissue.
Pafolacianine absorbs light in the near-infrared (NIR) region within a range of 760 nm to 785 nm with peak absorption of 776 nm and emits fluorescence within a range of 790 nm to 815 nm with peak emission of 796 nm.
12.2 Pharmacodynamics
Tumor to background ratios of fluorescence intensity changed with different mass doses studied in patients with ovarian cancer. High tumor to background ratio was observed with 0.025 mg/kg dose. CYTALUX exposure-response relationships and the time course of pharmacodynamic responses are unknown.
12.3 Pharmacokinetics
The mean C max of pafolacianine was 59.1 ± 5.94 ng/mL and AUC inf was 63.6 ± 12.6 ng.hr/mL.
Distribution
The mean volume of distribution (V z) is 17.1 ± 5.99 L, indicating distribution into tissues.
Plasma protein binding of pafolacianine is 93.7%. No notable partitioning into red blood cells has
been observed.
Elimination
The elimination half-life of pafolacianine is 0.44 ± 0.23 hours and mean plasma clearance is 28.6 ±
4.97 L/hr.
Metabolism
Pafolacianine sodium is not metabolized by cytochrome P450 (CYP) enzymes.
Excretion
Following a single IV infusion of radiolabeled pafolacianine sodium, approximately 35% of the dose
was recovered in urine (19.1%) and in feces (15.8%) after approximately 3-5 weeks.
Specific Populations
No clinically significant differences in pharmacokinetics of pafolacianine were identified based on age
18 to 89 years, weight 41.6 to 133.6 kg, mild to moderate renal impairment (CLcr 30 to 89 mL/min),
mild to moderate hepatic impairment (total bilirubin < 3 ULN and AST > ULN). The effect of severe
renal impairment (CLcr < 30 mL/min) and severe hepatic impairment (total bilirubin > 3 ULN and any
AST value) on the pharmacokinetics of pafolacianine have not been studied.
Drug Interaction Studies
No clinical studies evaluating the drug interaction potential of pafolacianine have been conducted.
In Vitro Studies
CYP Enzymes: Pafolacianine is not an inhibitor of CYPs 1A2, 2B6, 2C8, 2C9, 2C19 2D6, 3A4/5.
UDP-glucuronosyltransferase (UGT) Enzymes: Pafolacianine is not an inhibitor of UGT1A1.
Transporter Systems: Pafolacianine is a substrate for OATP1B1, OATP1B3, and OAT1. Pafolacianine
is not an inhibitor of OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1, MATE2-K, P-gp, or BCRP -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity studies of pafolacianine have been conducted.
Mutagenesis
No genotoxic hazards were identified when pafolacianine was evaluated in a standard testing battery
consisting of a bacterial reverse mutation assay, an in vitro micronucleus study conducted in Chinese
Hamster Ovary (CHO) cells, and a rat bone marrow micronucleus study.
Impairment of Fertility
Reproductive and developmental toxicity (fertility and embryonic development, pre- and postnatal
development) studies in animals have not been performed to evaluate the effects of pafolacianine on
fertility. -
14 CLINICAL STUDIES
14.1 Patients with Known or Suspected Ovarian Cancer
The safety and efficacy of CYTALUX were evaluated in a randomized, multicenter, open-label study (NCT03180307). The study enrolled 178 women with a diagnosis or high clinical suspicion of ovarian cancer scheduled to undergo primary surgical cytoreduction, interval debulking, or recurrent ovarian cancer surgery. One hundred and fifty women received CYTALUX (dosed at 0.025 mg/kg at least 1 hour before initiation of fluorescence imaging). Among them, 134 women underwent both normal light and CYTALUX evaluation (Intent-to-Image Set). The demographic characteristics were mean age 60 (range 33 to 81) years, 85% White, 4% Asian, 5% Black or African American, 3% American Indian or Alaska native, 3% other races, 11% Hispanic or Latino, and 2% unreported ethnicity.
Table 2 shows the proportion of patients with at least one evaluable ovarian cancer lesion confirmed by central histopathology that was detected with CYTALUX but not with normal light or palpation and not otherwise identified for resection prior to surgery. The detection performance met the pre-specified success threshold.
Table 2: Detection Proportion with CYTALUX but Not with Normal Light or Palpation in the Intent-To-Image Set
N=134 patients Patients with at least one confirmed ovarian cancer evaluable lesion
Number (n) 36 Proportion (%)
95% CI0.269 (26.9%)
(0.196*, 0.352)*The pre-specified lower bound of the 95% confidence interval (CI) was 0.10.
In 20.2% (95% CI: 13.7%, 28.0%) of patients, all lesions detected by CYTALUX alone were negative by central histopathology (false positive).
14.2 Patients with Known or Suspected Cancer in the Lung
The safety and efficacy of CYTALUX were evaluated in a randomized, multicenter, open-label study (NCT04241315). The study enrolled 140 patients scheduled to undergo thoracoscopic or open segmental or subsegmental resection for primary lung lesions that were either confirmed or suspected to be cancer. One hundred and twelve patients received CYTALUX (dosed at 0.025 mg/kg between 1 and 24 hours before initiation of fluorescence imaging). Among them, 100 patients underwent both normal light and CYTALUX evaluation (Intent-to-Image Set). The demographic characteristics were 61% female, mean age 66 (range 26 to 83) years, 88% White, 8% Black or African American, 2% Asian, 2% other races, 99% Not Hispanic or Latino, and 1% unreported ethnicity. Histopathology of the primary lung lesions in these 100 patients showed primary lung cancer in 65%, metastasis to the lung in 21%, benign lung lesions in 11%, and other/unknown cancer in 3%. Among the primary lung cancers, 86% were adenocarcinoma, 8% were squamous cell carcinoma, 3% were adeno-squamous carcinoma, and 3% were atypical carcinoid.
Table 3 shows the proportion of patients in whom at least one of the following clinically significant events (CSE) occurred with CYTALUX but not with normal light or palpation: localization of the primary lung lesion, whether benign or malignant (CSE A), and detection of one or more synchronous malignant lung lesions (CSE B). Synchronous lesions were not identified prior to surgery. The combined CSE A and CSE B detection performance met the pre-specified success threshold.
Table 3: Proportion of Clinically Significant Events Occurring with CYTALUX but Not with Normal Light or Palpation in the Intent-To-Image Set
N=100 patients Primary Lung Lesion (CSE A) Synchronous Malignant Lung Lesion (CSE B) Patients with CSE A and/or CSE B Number (n) 19* 8 24** Proportion (%)
95% CI0.19 (19%)
(0.118, 0.281)0.08 (8%)
(0.035, 0.152)0.24 (24%)
(0.160***, 0.336)* Including two subjects with benign primary lesions.
** Three patients had both primary lesion localization and synchronous malignant lesion detection, resulting in 27 events in 24 unique patients.
*** The pre-specified lower bound of the 95% confidence interval (CI) was 0.10.
CYTALUX did not identify the primary lung lesion in 23% (95% CI:15%, 32%) of patients. Among the 20 patients who had synchronous lesions detected only by CYTALUX, 12 patients had only benign synchronous lesions.
The depth of primary lung lesions detected by CYTALUX ranged from 0 to 38 mm from the lung surface (mean depth 6 mm).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
CYTALUX (pafolacianine) injection, 3.2 mg /1.6 mL (2 mg/mL), is a dark bluish green, clear aqueous
solution packaged in a sealed amber glass single-dose vial. It is supplied in a carton containing 10
vials (NDC: 81052-138-10) individually packaged.Storage and Handling
Store vials in their original cartons to protect from light.CYTALUX may be stored according to the table below. If CYTALUX vial is not used within the maximum single period at either room temperature or under refrigerated conditions the vial may be refrozen up to three time.
Storage Condition
Condition Temperature Range
Maximum Single Period Permissible Freeze-Thaw Cycles
Frozen
-25°C to -15°C (-13°F to 5°F)
Until Expiration Date
Not applicable
Refrigeration
2°C to 8°C (36°F to 46°F)
Up to 30 days Three
Room Temperature
20°C to 25°C (68°F to 77°F)
Up to 24 hours
Three
Do not use the product past the expiration date
-
17 PATIENT COUNSELING INFORMATION
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to contact their healthcare
provider with a known or suspected pregnancy
[ see Warnings and Precautions ( 5.3) and Use In Specific Populations ( 8.1) ].Folate Supplements Usage
Inform patients that folic acid may reduce the detection of cancer tissue with CYTALUX. Advise the
patient to stop taking folate, folic acid, or folate-containing supplements 48 hours before
administration of CYTALUX [ see Dosage and Administration ( 2.1) and Drug Interactions ( 7) ]. - SPL UNCLASSIFIED SECTION
-
PACKAGING - PRINCIPAL DISPLAY PANEL
10 Vial Carton
NDC: 81052-138-10
cytalux™
(pafolacianine) injection3.2 mg / 1.6 mL (2 mg/mL)
10 x 1.6 mL Single-Dose Vials. Discard Unused Portion
For Intravenous Infusion After DilutionStore vials in original carton to protect from light. Store frozen at
-25° to -15°C (-13° to 5°F) until expiration date. Once thawed, vialsmay be stored refrigerated at 2° to 8°C (36° to 46°F) up to 30 days
or at room temperature at 20° to 25°C (68° to 77°F) up to 24 hours.
Rx only
ON TARGET LABORATORIES
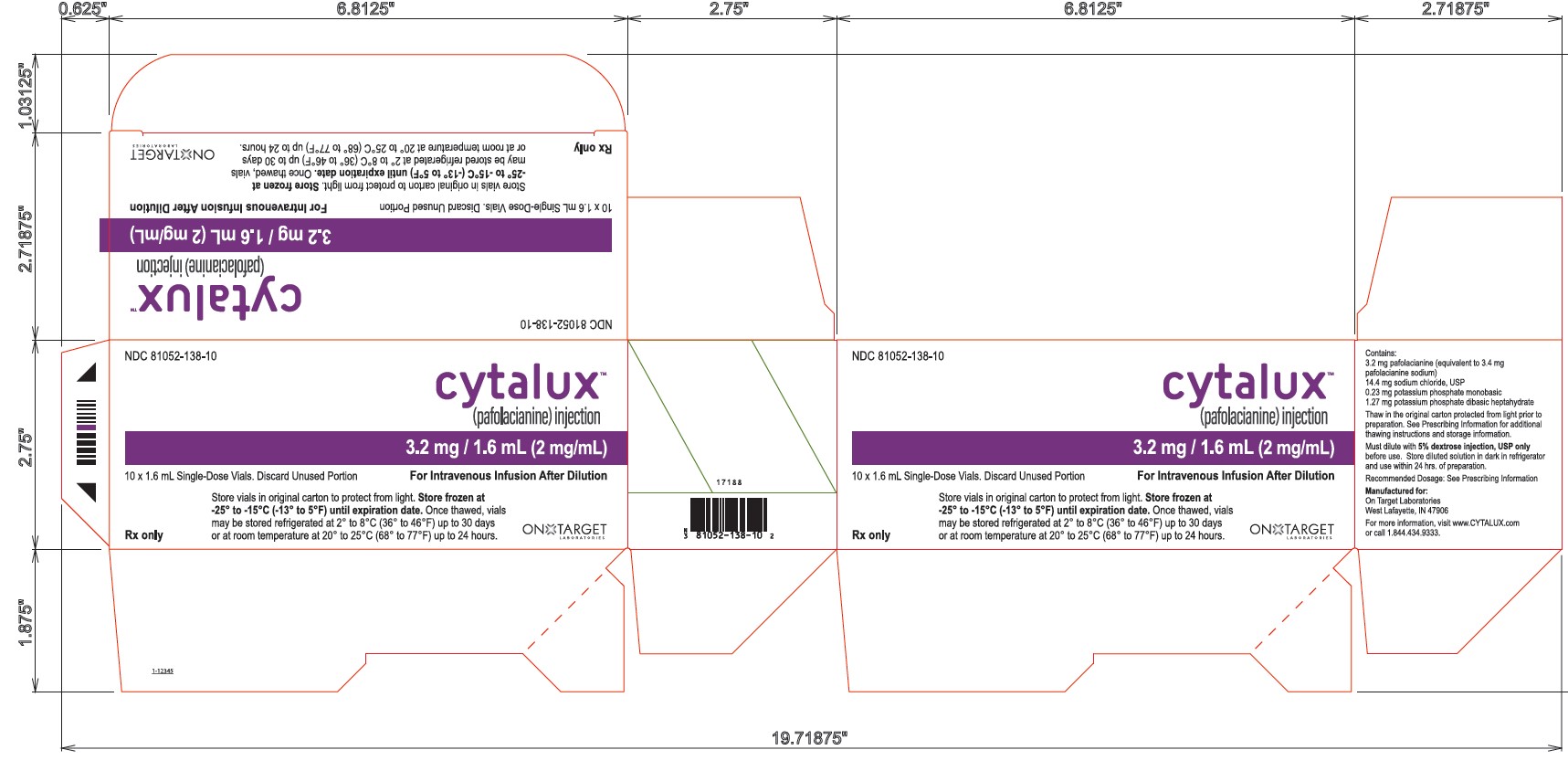
Vial Label
NDC: 81052-138-10
Sterile
cytalux™
(pafolacianine) injection3.2 mg / 1.6 mL
(2 mg/mL)For Intravenous Infusion After Dilution
Discard Unused Portion
Single-Dose Vial
Rx only
ON TARGET LABORATORIES
17727
LOT:
EXP: YYYY/MM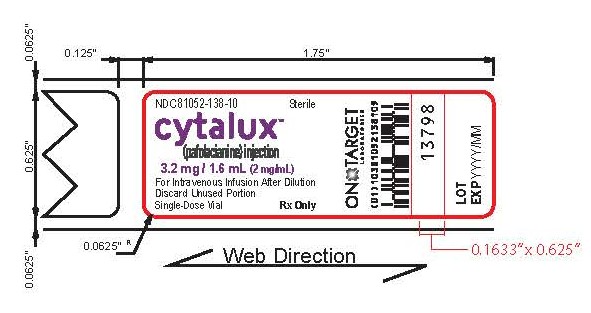
Single Vial Carton Label
NDC: 81052-138-01
cytalux™
(pafolacianine) injection3.2 mg / 1.6 mL
(2 mg/mL)
For Intravenous Infusion After DilutionStore vials in original carton to
protect from light. Store frozen at
-25° to -15°C (-13° to 5°F) until
expiration date. Once thawed, vials
may be stored refrigerated at 2° to 8°C
(36° to 46°F) up to 30 days or at room
temperature at 20° to 25°C (68° to
77°F) up to 24 hours.Rx only
ON TARGET LABORATORIES
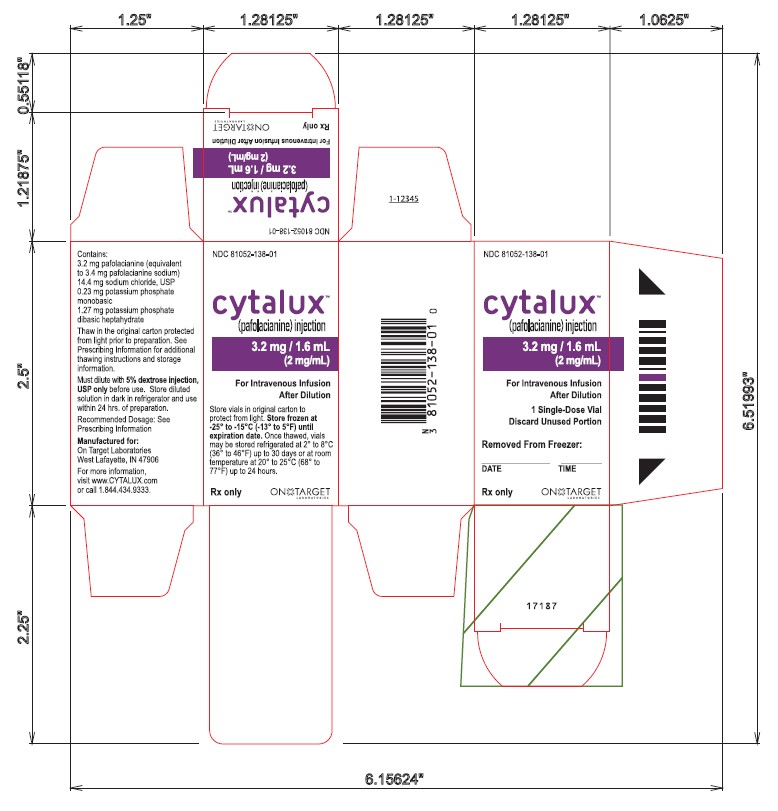
-
INGREDIENTS AND APPEARANCE
CYTALUX
pafolacianine injection injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 81052-138 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PAFOLACIANINE SODIUM (UNII: 4HUF3V875C) (PAFOLACIANINE - UNII:F7BD3Z4X8L) PAFOLACIANINE 3.2 mg in 1.6 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color green (Dark Bluish) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 81052-138-10 10 in 1 CARTON 11/29/2021 1 1 in 1 CARTON 1 NDC: 81052-138-01 1.6 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214907 11/29/2021 Labeler - On Target Laboratories, Inc. (968729181)
Trademark Results [Cytalux]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYTALUX 88890150 not registered Live/Pending |
ON TARGET LABORATORIES, INC. 2020-04-27 |
 CYTALUX 88890147 not registered Live/Pending |
ON TARGET LABORATORIES, INC. 2020-04-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.