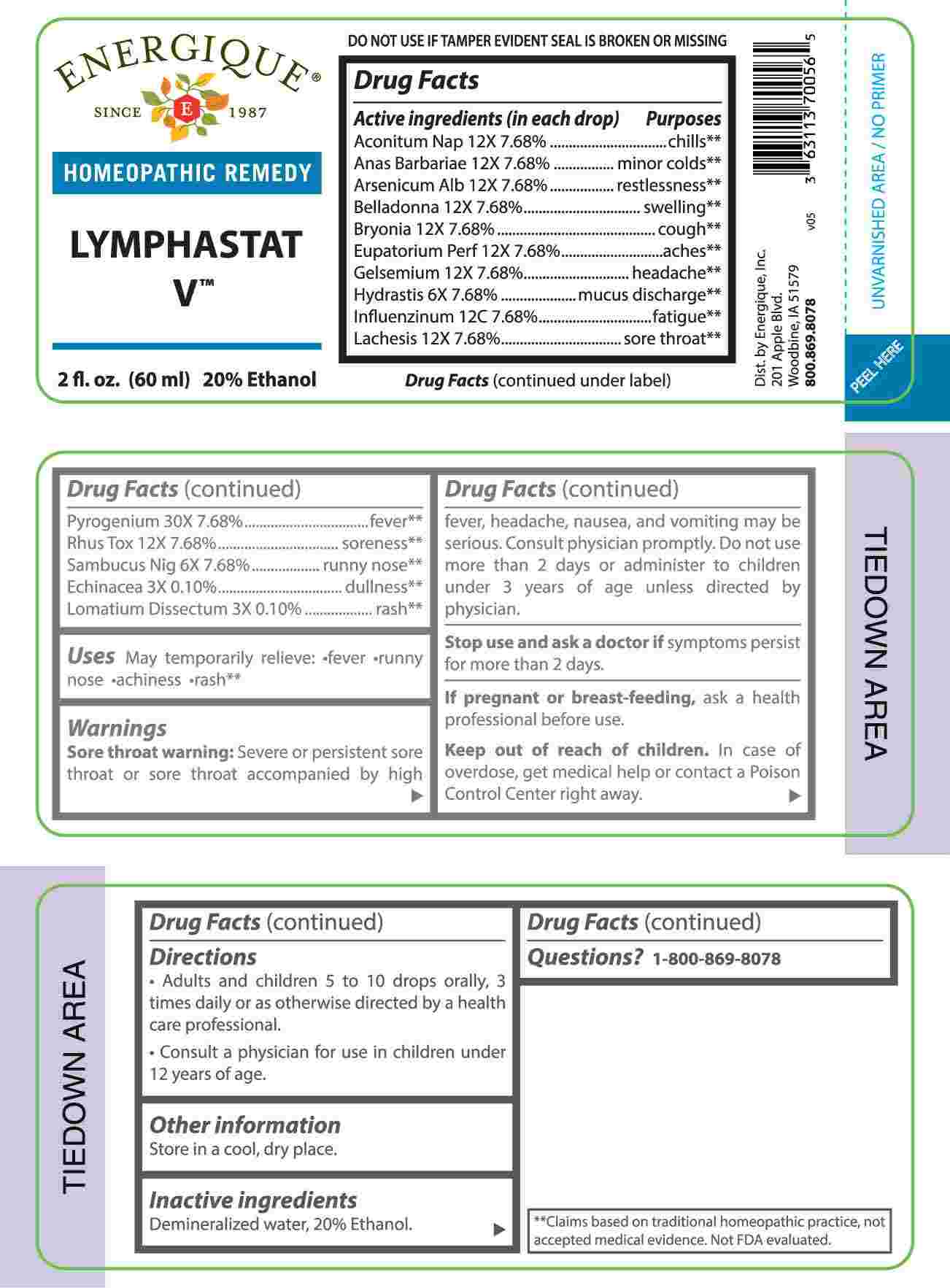
LYMPHASTAT V (echinacea (angustifolia), lomatium dissectum, hydrastis canadensis, sambucus nigra, aconitum napellus, anas barbariae, hepatis et cordis extractum, arsenicum album, belladonna, bryonia- alba, eupatorium perfoliatum, gelsemium sempervirens, lachesis mutus, rhus tox, pyrogenium, influenzinum liquid
Lymphastat V by
Drug Labeling and Warnings
Lymphastat V by is a Homeopathic medication manufactured, distributed, or labeled by Energique, Inc., Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENTS:
(in each drop) Aconitum Napellus 12X 7.68%, Anas Barbariae 12X 7.68%, Arsenicum Album 12X 7.68%, Belladonna 12X 7.68%, Bryonia 12X 7.68%, Eupatorium Perfoliatum 12X 7.68%, Gelsemium Sempervirens 12X 7.68%, Hydrastis Canadensis 6X 7.68%, Influenzinum 12C 7.68%, Lachesis Mutus 12X 7.68%, Pyrogenium 30X 7.68%, Rhus Tox 12X 7.68%, Sambucus Nigra 6X 7.68%, Echinacea 3X 0.10, Lomatium Dissectum 3X 0.10%.
-
PURPOSE:
Aconitum Napellus - chills,** Anas Barbariae – minor colds,** Arsenicum Album - restlessness,** Belladonna - swelling,** Bryonia - cough,** Eupatorium Perfoliatum - aches,** Gelsemium Sempervirens - headache,** Hydrastis Canadensis – mucus discharge,** Influenzinum - fatigue,** Lachesis Mutus – sore throat,** Pyrogenium - fever,** Rhus Tox - soreness,** Sambucus Nigra – runny nose,** Echinacea - dullness,** Lomatium Dissectum – rash**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- USES:
-
WARNINGS:
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult physician promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by physician.
Stop use and ask a doctor if symptoms persist for more than 2 days.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DO NOT USE IF TAMPER EVIDENT SEAL IS BROKEN OR MISSING
Store in a cool, dry place.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
LYMPHASTAT V
echinacea (angustifolia), lomatium dissectum, hydrastis canadensis, sambucus nigra, aconitum napellus, anas barbariae, hepatis et cordis extractum, arsenicum album, belladonna, bryonia (alba), eupatorium perfoliatum, gelsemium sempervirens, lachesis mutus, rhus tox, pyrogenium, influenzinum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 44911-0721 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ECHINACEA ANGUSTIFOLIA WHOLE (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA WHOLE 3 [hp_X] in 1 mL LOMATIUM DISSECTUM ROOT (UNII: 5329928G5N) (LOMATIUM DISSECTUM ROOT - UNII:5329928G5N) LOMATIUM DISSECTUM ROOT 3 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) (SAMBUCUS NIGRA FLOWERING TOP - UNII:CT03BSA18U) SAMBUCUS NIGRA FLOWERING TOP 6 [hp_X] in 1 mL ACONITUM NAPELLUS WHOLE (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS WHOLE 12 [hp_X] in 1 mL CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 12 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 1 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 12 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 12 [hp_X] in 1 mL EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 12 [hp_X] in 1 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 12 [hp_X] in 1 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 12 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 12 [hp_X] in 1 mL RANCID BEEF (UNII: 29SUH5R3HU) (RANCID BEEF - UNII:29SUH5R3HU) RANCID BEEF 30 [hp_X] in 1 mL INFLUENZA A VIRUS WHOLE (UNII: R9HH0NDE2E) (INFLUENZA A VIRUS WHOLE - UNII:R9HH0NDE2E) INFLUENZA A VIRUS WHOLE 12 [hp_C] in 1 mL INFLUENZA A VIRUS A/DARWIN/6/2021 (H3N2) WHOLE (UNII: PMY5J4Z7LS) (INFLUENZA A VIRUS A/DARWIN/6/2021 (H3N2) WHOLE - UNII:PMY5J4Z7LS) INFLUENZA A VIRUS A/DARWIN/6/2021 (H3N2) WHOLE 12 [hp_C] in 1 mL INFLUENZA B VIRUS B/AUSTRIA/1359417/2021 BVR-26 WHOLE (UNII: CYK5P89K8X) (INFLUENZA B VIRUS B/AUSTRIA/1359417/2021 BVR-26 WHOLE - UNII:CYK5P89K8X) INFLUENZA B VIRUS B/AUSTRIA/1359417/2021 BVR-26 WHOLE 12 [hp_C] in 1 mL INFLUENZA B VIRUS B/PHUKET/3073/2013 BVR-1B WHOLE (UNII: PS2DCE2WVF) (INFLUENZA B VIRUS B/PHUKET/3073/2013 BVR-1B WHOLE - UNII:PS2DCE2WVF) INFLUENZA B VIRUS B/PHUKET/3073/2013 BVR-1B WHOLE 12 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44911-0721-1 60 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 09/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/30/2024 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0721) , api manufacture(44911-0721) , label(44911-0721) , pack(44911-0721)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.