PERCOCET- oxycodone hydrochloride and acetaminophen tablet
PERCOCET by
Drug Labeling and Warnings
PERCOCET by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.
-
DESCRIPTION
Each tablet, for oral administration, contains oxycodone hydrochloride and acetaminophen in the following strengths:
Oxycodone Hydrochloride, USP 2.5 mg*
Acetaminophen, USP 325 mg
*2.5 mg oxycodone HCl is equivalent to 2.2409 mg of oxycodone.Oxycodone Hydrochloride, USP 5 mg*
Acetaminophen, USP 325 mg
*5 mg oxycodone HCl is equivalent to 4.4815 mg of oxycodone.Oxycodone Hydrochloride, USP 7.5 mg*
Acetaminophen, USP 325 mg
*7.5 mg oxycodone HCl is equivalent to 6.7228 mg of oxycodone.Oxycodone Hydrochloride, USP 7.5 mg*
Acetaminophen, USP 500 mg
*7.5 mg oxycodone HCl is equivalent to 6.7228 mg of oxycodone.Oxycodone Hydrochloride, USP 10 mg*
Acetaminophen, USP 325 mg
*10 mg oxycodone HCl is equivalent to 8.9637 mg of oxycodone.Oxycodone Hydrochloride, USP 10 mg*
Acetaminophen, USP 650 mg
*10 mg oxycodone HCl is equivalent to 8.9637 mg of oxycodone.All strengths of PERCOCET also contain the following inactive ingredients: Colloidal silicon dioxide, croscarmellose sodium, crospovidone, microcrystalline cellulose, povidone, pregelatinized cornstarch, and stearic acid. In addition, the 2.5 mg/325 mg strength contains FD&C Red No. 40 Aluminum Lake and the 5 mg/325 mg strength contains FD&C Blue No. 1 Aluminum Lake. The 7.5 mg/325 mg and the 7.5 mg/500 mg strengths contain FD&C Yellow No. 6 Aluminum Lake. The 10 mg/325 mg and the 10 mg/650 mg strengths contain D&C Yellow No. 10 Aluminum Lake.
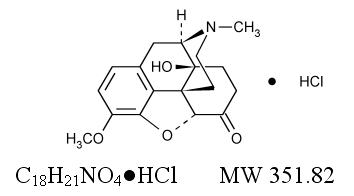
Oxycodone, 14-hydroxydihydrocodeinone, is a semisynthetic opioid analgesic which occurs as a white, odorless, crystalline powder having a saline, bitter taste. The molecular formula for oxycodone hydrochloride is C18H21NO4HCl and the molecular weight 351.82. It is derived from the opium alkaloid thebaine, and may be represented by the following structural formula:
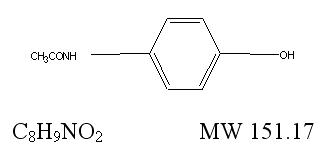
Acetaminophen, 4’-hydroxyacetanilide, is a non-opiate, non-salicylate analgesic and antipyretic which occurs as a white, odorless, crystalline powder, possessing a slightly bitter taste. The molecular formula for acetaminophen is C8H9NO2 and the molecular weight is 151.16. It may be represented by the following structural formula:
-
CLINICAL PHARMACOLOGY
Central Nervous System
Oxycodone is a semisynthetic pure opioid agonist whose principal therapeutic action is analgesia. Other pharmacological effects of oxycodone include anxiolysis, euphoria and feelings of relaxation. These effects are mediated by receptors (notably µ and κ) in the central nervous system for endogenous opioid-like compounds such as endorphins and enkephalins. Oxycodone produces respiratory depression through direct activity at respiratory centers in the brain stem and depresses the cough reflex by direct effect on the center of the medulla.
Acetaminophen is a non-opiate, non-salicylate analgesic and antipyretic. The site and mechanism for the analgesic effect of acetaminophen has not been determined. The antipyretic effect of acetaminophen is accomplished through the inhibition of endogenous pyrogen action on the hypothalamic heat-regulating centers.
Gastrointestinal Tract and Other Smooth Muscle
Oxycodone reduces motility by increasing smooth muscle tone in the stomach and duodenum. In the small intestine, digestion of food is delayed by decreases in propulsive contractions. Other opioid effects include contraction of biliary tract smooth muscle, spasm of the Sphincter of Oddi, increased ureteral and bladder sphincter tone, and a reduction in uterine tone.
Cardiovascular System
Oxycodone may produce a release of histamine and may be associated with orthostatic hypotension, and other symptoms, such as pruritus, flushing, red eyes, and sweating.
Pharmacokinetics
Absorption and Distribution
The mean absolute oral bioavailability of oxycodone in cancer patients was reported to be about 87%. Oxycodone has been shown to be 45% bound to human plasma proteins in vitro. The volume of distribution after intravenous administration is 211.9 ±186.6 L.
Absorption of acetaminophen is rapid and almost complete from the GI tract after oral administration. With overdosage, absorption is complete in 4 hours. Acetaminophen is relatively uniformly distributed throughout most body fluids. Binding of the drug to plasma proteins is variable; only 20% to 50% may be bound at the concentrations encountered during acute intoxication.
Metabolism and Elimination
A high portion of oxycodone is N-dealkylated to noroxycodone during first-pass metabolism. Oxymorphone, is formed by the O-demethylation of oxycodone. The metabolism of oxycodone to oxymorphone is catalyzed by CYP2D6. Free and conjugated noroxycodone, free and conjugated oxycodone, and oxymorphone are excreted in human urine following a single oral dose of oxycodone. Approximately 8% to 14% of the dose is excreted as free oxycodone over 24 hours after administration. Following a single, oral dose of oxycodone, the mean ± SD elimination half-life is 3.51 ± 1.43 hours.
Acetaminophen is metabolized in the liver via cytochrome P450 microsomal enzyme. About 80-85% of the acetaminophen in the body is conjugated principally with glucuronic acid and to a lesser extent with sulfuric acid and cysteine. After hepatic conjugation, 90 to 100% of the drug is recovered in the urine with in the first day.
About 4% of acetaminophen is metabolized via cytochrome P450 oxidase to a toxic metabolite which is further detoxified by conjugation with glutathione, present in a fixed amount. It is believed that the toxic metabolite NAPQI (N acetyl-p-benzoquinoneimine, N-acetylimidoquinone) is responsible for liver necrosis. High doses of acetaminophen may deplete the glutathione stores so that inactivation of the toxic metabolite is decreased. At high doses, the capacity of metabolic pathways for conjugation with glucuronic acid and sulfuric acid may be exceeded, resulting in increased metabolism of acetaminophen by alternate pathways.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
PERCOCET tablets should not be administered to patients with known hypersensitivity to oxycodone, acetaminophen, or any other component of this product.
Oxycodone is contraindicated in any situation where opioids are contraindicated including patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment) and patients with acute or severe bronchial asthma or hypercarbia. Oxycodone is contraindicated in the setting of suspected or known paralytic ileus.
-
WARNINGS
Misuse, Abuse and Diversion of Opioids
Oxycodone is an opioid agonist of the morphine-type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Oxycodone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing PERCOCET tablets in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion. Concerns about misuse, addiction, and diversion should not prevent the proper management of pain.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Administration of PERCOCET (Oxycodone and Acetaminophen Tablets, USP) tablets should be closely monitored for the following potentially serious adverse reactions and complications:
Respiratory Depression
Respiratory depression is a hazard with the use of oxycodone, one of the active ingredients in PERCOCET tablets, as with all opioid agonists. Elderly and debilitated patients are at particular risk for respiratory depression as are non-tolerant patients given large initial doses of oxycodone or when oxycodone is given in conjunction with other agents that depress respiration. Oxycodone should be used with extreme caution in patients with acute asthma, chronic obstructive pulmonary disorder (COPD), cor pulmonale, or preexisting respiratory impairment. In such patients, even usual therapeutic doses of oxycodone may decrease respiratory drive to the point of apnea. In these patients alternative non-opioid analgesics should be considered, and opioids should be employed only under careful medical supervision at the lowest effective dose.
In case of respiratory depression, a reversal agent such as naloxone hydrochloride may be utilized (see OVERDOSAGE).
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, and may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Oxycodone produces effects on pupillary response and consciousness which may obscure neurologic signs of worsening in patients with head injuries.
Hypotensive Effect
Oxycodone may cause severe hypotension particularly in individuals whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs which compromise vasomotor tone such as phenothiazines. Oxycodone, like all opioid analgesics of the morphine-type, should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure. Oxycodone may produce orthostatic hypotension in ambulatory patients.
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
Hypersensitivity / anaphylaxis
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs including swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue PERCOCET immediately and seek medical care if they experience these symptoms. Do not prescribe PERCOCET for patients with acetaminophen allergy.
-
PRECAUTIONS
General
Opioid analgesics should be used with caution when combined with CNS depressant drugs, and should be reserved for cases where the benefits of opioid analgesia outweigh the known risks of respiratory depression, altered mental state, and postural hypotension.
Acute Abdominal Conditions
The administration of PERCOCET (Oxycodone and Acetaminophen Tablets, USP) or other opioids may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
PERCOCET tablets should be given with caution to patients with CNS depression, elderly or debilitated patients, patients with severe impairment of hepatic, pulmonary, or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy, urethral stricture, acute alcoholism, delirium tremens, kyphoscoliosis with respiratory depression, myxedema, and toxic psychosis.
PERCOCET tablets may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Oxycodone may aggravate convulsions in patients with convulsive disorders, and all opioids may induce or aggravate seizures in some clinical settings.
Following administration of PERCOCET tablets, anaphylactic reactions have been reported in patients with a known hypersensitivity to codeine, a compound with a structure similar to morphine and oxycodone. The frequency of this possible cross-sensitivity is unknown.
Interactions with Other CNS Depressants
Patients receiving other opioid analgesics, general anesthetics, phenothiazines, other tranquilizers, centrally-acting anti-emetics, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with PERCOCET tablets may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Interactions with Mixed Agonist/Antagonist Opioid Analgesics
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, and butorphanol) should be administered with caution to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as oxycodone. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of oxycodone and/or may precipitate withdrawal symptoms in these patients.
Ambulatory Surgery and Postoperative Use
Oxycodone and other morphine-like opioids have been shown to decrease bowel motility. Ileus is a common postoperative complication, especially after intra-abdominal surgery with use of opioid analgesia. Caution should be taken to monitor for decreased bowel motility in postoperative patients receiving opioids. Standard supportive therapy should be implemented.
Use in Pancreatic/Biliary Tract Disease
Oxycodone may cause spasm of the Sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids like oxycodone may cause increases in the serum amylase level.
Tolerance and Physical Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
In general, opioids should not be abruptly discontinued (see DOSAGE AND ADMINISTRATION: Cessation of Therapy).
Information for Patients/Caregivers
The following information should be provided to patients receiving PERCOCET tablets by their physician, nurse, pharmacist, or caregiver:
- Do not take PERCOCET if you are allergic to any of its ingredients.
- If you develop signs of allergy such as a rash or difficulty breathing stop taking PERCOCET and contact your healthcare provider immediately.
- Do not take more than 4000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
- Patients should be aware that PERCOCET tablets contain oxycodone, which is a morphine-like substance.
- Patients should be instructed to keep PERCOCET tablets in a secure place out of the reach of children. In the case of accidental ingestions, emergency medical care should be sought immediately.
- When PERCOCET tablets are no longer needed, the unused tablets should be destroyed by flushing down the toilet.
- Patients should be advised not to adjust the medication dose themselves. Instead, they must consult with their prescribing physician.
- Patients should be advised that PERCOCET tablets may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating heavy machinery).
- Patients should not combine PERCOCET tablets with alcohol, opioid analgesics, tranquilizers, sedatives, or other CNS depressants unless under the recommendation and guidance of a physician. When co-administered with another CNS depressant, PERCOCET tablets can cause dangerous additive central nervous system or respiratory depression, which can result in serious injury or death.
- The safe use of PERCOCET tablets during pregnancy has not been established; thus, women who are planning to become pregnant or are pregnant should consult with their physician before taking PERCOCET tablets.
- Nursing mothers should consult with their physicians about whether to discontinue nursing or discontinue PERCOCET tablets because of the potential for serious adverse reactions to nursing infants.
- Patients who are treated with PERCOCET tablets for more than a few weeks should be advised not to abruptly discontinue the medication. Patients should consult with their physician for a gradual discontinuation dose schedule to taper off the medication.
- Patients should be advised that PERCOCET tablets are a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
Laboratory Tests
Although oxycodone may cross-react with some drug urine tests, no available studies were found which determined the duration of detectability of oxycodone in urine drug screens. However, based on pharmacokinetic data, the approximate duration of detectability for a single dose of oxycodone is roughly estimated to be one to two days following drug exposure.
Urine testing for opiates may be performed to determine illicit drug use and for medical reasons such as evaluation of patients with altered states of consciousness or monitoring efficacy of drug rehabilitation efforts. The preliminary identification of opiates in urine involves the use of an immunoassay screening and thin-layer chromatography (TLC). Gas chromatography/mass spectrometry (GC/MS) may be utilized as a third-stage identification step in the medical investigational sequence for opiate testing after immunoassay and TLC. The identities of 6-keto opiates (e.g., oxycodone) can further be differentiated by the analysis of their methoxime-trimethylsilyl (MO-TMS) derivative.
Drug/Drug Interactions with Oxycodone
Opioid analgesics may enhance the neuromuscular-blocking action of skeletal muscle relaxants and produce an increase in the degree of respiratory depression.
Patients receiving CNS depressants such as other opioid analgesics, general anesthetics, phenothiazines, other tranquilizers, centrally-acting anti-emetics, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with PERCOCET tablets may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced. The concurrent use of anticholinergics with opioids may produce paralytic ileus.
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, naltrexone, and butorphanol) should be administered with caution to a patient who has received or is receiving a pure opioid agonist such as oxycodone. These agonist/antagonist analgesics may reduce the analgesic effect of oxycodone or may precipitate withdrawal symptoms.
Drug/Drug Interactions with Acetaminophen
Alcohol, ethyl: Hepatotoxicity has occurred in chronic alcoholics following various dose levels (moderate to excessive) of acetaminophen.
Anticholinergics: The onset of acetaminophen effect may be delayed or decreased slightly, but the ultimate pharmacological effect is not significantly affected by anticholinergics.
Oral Contraceptives: Increase in glucuronidation resulting in increased plasma clearance and a decreased half-life of acetaminophen.
Charcoal (activated): Reduces acetaminophen absorption when administered as soon as possible after overdose.
Beta Blockers (Propanolol): Propanolol appears to inhibit the enzyme systems responsible for the glucuronidation and oxidation of acetaminophen. Therefore, the pharmacologic effects of acetaminophen may be increased.
Loop diuretics: The effects of the loop diuretic may be decreased because acetaminophen may decrease renal prostaglandin excretion and decrease plasma renin activity.
Lamotrigine: Serum lamotrigine concentrations may be reduced, producing a decrease in therapeutic effects.
Probenecid: Probenecid may increase the therapeutic effectiveness of acetaminophen slightly.
Zidovudine: The pharmacologic effects of zidovudine may be decreased because of enhanced non-hepatic or renal clearance of zidovudine.
Drug/Laboratory Test Interactions
Depending on the sensitivity/specificity and the test methodology, the individual components of PERCOCET (Oxycodone and Acetaminophen Tablets, USP) may cross-react with assays used in the preliminary detection of cocaine (primary urinary metabolite, benzoylecgonine) or marijuana (cannabinoids) in human urine. A more specific alternate chemical method must be used in order to obtain a confirmed analytical result. The preferred confirmatory method is gas chromatography/mass spectrometry (GC/MS). Moreover, clinical considerations and professional judgment should be applied to any drug-of-abuse test result, particularly when preliminary positive results are used.
Acetaminophen may interfere with home blood glucose measurement systems; decreases of >20% in mean glucose values may be noted. This effect appears to be drug, concentration and system dependent.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal studies to evaluate the carcinogenic potential of oxycodone and acetaminophen have not been performed.
Mutagenesis
The combination of oxycodone and acetaminophen has not been evaluated for mutagenicity. Oxycodone alone was negative in a bacterial reverse mutation assay (Ames), an in vitro chromosome aberration assay with human lymphocytes without metabolic activation and an in vivo mouse micronucleus assay. Oxycodone was clastogenic in the human lymphocyte chromosomal assay in the presence of metabolic activation and in the mouse lymphoma assay with or without metabolic activation.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproductive studies have not been conducted with PERCOCET. It is also not known whether PERCOCET can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. PERCOCET should not be given to a pregnant woman unless in the judgment of the physician, the potential benefits outweigh the possible hazards
Labor and Delivery
PERCOCET tablets are not recommended for use in women during and immediately prior to labor and delivery due to its potential effects on respiratory function in the newborn.
Nursing Mothers
Ordinarily, nursing should not be undertaken while a patient is receiving PERCOCET tablets because of the possibility of sedation and/or respiratory depression in the infant. Oxycodone is excreted in breast milk in low concentrations, and there have been rare reports of somnolence and lethargy in babies of nursing mothers taking an oxycodone/acetaminophen product. Acetaminophen is also excreted in breast milk in low concentrations.
Geriatric Use
Special precaution should be given when determining the dosing amount and frequency of PERCOCET tablets for geriatric patients, since clearance of oxycodone may be slightly reduced in this patient population when compared to younger patients.
-
ADVERSE REACTIONS
Serious adverse reactions that may be associated with PERCOCET tablet use include respiratory depression, apnea, respiratory arrest, circulatory depression, hypotension, and shock (see OVERDOSAGE).
The most frequently observed non-serious adverse reactions include lightheadedness, dizziness, drowsiness or sedation, nausea, and vomiting. These effects seem to be more prominent in ambulatory than in nonambulatory patients, and some of these adverse reactions may be alleviated if the patient lies down. Other adverse reactions include euphoria, dysphoria, constipation, and pruritus.
Hypersensitivity reactions may include: Skin eruptions, urticarial, erythematous skin reactions. Hematologic reactions may include: Thrombocytopenia, neutropenia, pancytopenia, hemolytic anemia. Rare cases of agranulocytosis has likewise been associated with acetaminophen use. In high doses, the most serious adverse effect is a dose-dependent, potentially fatal hepatic necrosis. Renal tubular necrosis and hypoglycemic coma also may occur.
Other adverse reactions obtained from postmarketing experiences with PERCOCET tablets are listed by organ system and in decreasing order of severity and/or frequency as follows:
Body as a Whole
Anaphylactoid reaction, allergic reaction, malaise, asthenia, fatigue, chest pain, fever, hypothermia, thirst, headache, increased sweating, accidental overdose, non-accidental overdose
Cardiovascular
Hypotension, hypertension, tachycardia, orthostatic hypotension, bradycardia, palpitations, dysrhythmias
Central and Peripheral Nervous System
Stupor, tremor, paraesthesia, hypoaesthesia, lethargy, seizures, anxiety, mental impairment, agitation, cerebral edema, confusion, dizziness
Gastrointestinal
Dyspepsia, taste disturbances, abdominal pain, abdominal distention, sweating increased, diarrhea, dry mouth, flatulence, gastro-intestinal disorder, nausea, vomiting, pancreatitis, intestinal obstruction, ileus
Hepatic
Transient elevations of hepatic enzymes, increase in bilirubin, hepatitis, hepatic failure, jaundice, hepatotoxicity, hepatic disorder
Hypersensitivity
Acute anaphylaxis, angioedema, asthma, bronchospasm, laryngeal edema, urticaria, anaphylactoid reaction
Psychiatric
Drug dependence, drug abuse, insomnia, confusion, anxiety, agitation, depressed level of consciousness, nervousness, hallucination, somnolence, depression, suicide
-
DRUG ABUSE AND DEPENDENCE
PERCOCET tablets are a Schedule II controlled substance. Oxycodone is a mu-agonist opioid with an abuse liability similar to morphine. Oxycodone, like morphine and other opioids used in analgesia, can be abused and is subject to criminal diversion.
Drug addiction is defined as an abnormal, compulsive use, use for non-medical purposes of a substance despite physical, psychological, occupational or interpersonal difficulties resulting from such use, and continued use despite harm or risk of harm. Drug addiction is a treatable disease, utilizing a multi-disciplinary approach, but relapse is common. Opioid addiction is relatively rare in patients with chronic pain but may be more common in individuals who have a past history of alcohol or substance abuse or dependence. Pseudoaddiction refers to pain relief seeking behavior of patients whose pain is poorly managed. It is considered an iatrogenic effect of ineffective pain management. The health care provider must assess continuously the psychological and clinical condition of a pain patient in order to distinguish addiction from pseudoaddiction and thus, be able to treat the pain adequately.
Physical dependence on a prescribed medication does not signify addiction. Physical dependence involves the occurrence of a withdrawal syndrome when there is sudden reduction or cessation in drug use or if an opiate antagonist is administered. Physical dependence can be detected after a few days of opioid therapy. However, clinically significant physical dependence is only seen after several weeks of relatively high dosage therapy. In this case, abrupt discontinuation of the opioid may result in a withdrawal syndrome. If the discontinuation of opioids is therapeutically indicated, gradual tapering of the drug over a 2-week period will prevent withdrawal symptoms. The severity of the withdrawal syndrome depends primarily on the daily dosage of the opioid, the duration of therapy and medical status of the individual.
The withdrawal syndrome of oxycodone is similar to that of morphine. This syndrome is characterized by yawning, anxiety, increased heart rate and blood pressure, restlessness, nervousness, muscle aches, tremor, irritability, chills alternating with hot flashes, salivation, anorexia, severe sneezing, lacrimation, rhinorrhea, dilated pupils, diaphoresis, piloerection, nausea, vomiting, abdominal cramps, diarrhea and insomnia, and pronounced weakness and depression.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor Shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated infection.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Oxycodone, like other opioids, has been diverted for non-medical use. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Like other opioid medications, PERCOCET tablets are subject to the Federal Controlled Substances Act. After chronic use, PERCOCET tablets should not be discontinued abruptly when it is thought that the patient has become physically dependent on oxycodone.
-
OVERDOSAGE
Following an acute overdosage, toxicity may result from the oxycodone or the acetaminophen.
Signs and Symptoms
Toxicity from oxycodone poisoning includes the opioid triad of: pinpoint pupils, depression of respiration, and loss of consciousness. Serious overdosage with oxycodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest, and death may occur.
In acetaminophen overdosage: dose-dependent potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and coagulation defects may also occur.Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment
A single or multiple drug overdose with oxycodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended. Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered.
Oxycodone
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The opioid antagonist naloxone hydrochloride is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to opioids, including oxycodone. Since the duration of action of oxycodone may exceed that of the antagonist, the patient should be kept under continued surveillance, and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. An opioid antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
Acetaminophen
Gastric decontamination with activated charcoal should be administered just prior to N-acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatotoxicity; acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication.
-
DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to the severity of the pain and the response of the patient. It may occasionally be necessary to exceed the usual dosage recommended below in cases of more severe pain or in those patients who have become tolerant to the analgesic effect of opioids. If pain is constant, the opioid analgesic should be given at regular intervals on an around-the-clock schedule. PERCOCET tablets are given orally.
PERCOCET 2.5 mg/325 mg
The usual adult dosage is one or 2 tablets every 6 hours. The total daily dose of acetaminophen should not exceed 4 grams.PERCOCET 5 mg/325 mg; PERCOCET 7.5 mg/500 mg; PERCOCET 10 mg/650 mg
The usual adult dosage is one tablet every 6 hours as needed for pain. The total daily dose of acetaminophen should not exceed 4 grams.PERCOCET 7.5 mg/325 mg; PERCOCET 10 mg/325 mg
The usual adult dosage is one tablet every 6 hours as needed for pain. The total daily dose of acetaminophen should not exceed 4 grams.Strength Maximal Daily Dose PERCOCET 2.5 mg/325 mg 12 Tablets PERCOCET 5 mg/325 mg 12 Tablets PERCOCET 7.5 mg/325 mg 8 Tablets PERCOCET 7.5 mg/500 mg 8 Tablets PERCOCET 10 mg/325 mg 6 Tablets PERCOCET 10 mg/650 mg 6 Tablets -
HOW SUPPLIED
PERCOCET (Oxycodone and Acetaminophen Tablets, USP) is supplied as follows:
2.5 mg/325 mg
Pink, oval, tablet debossed with “PERCOCET” on one side and “2.5” on the other.
Bottles of 100 NDC: 63481-627-705 mg/325 mg
Blue, round, tablet, debossed with “PERCOCET” and “5” on one side and bisect on the other.
Bottles of 100 NDC: 63481-623-70
Bottles of 500 NDC: 63481-623-85
Unit dose package of 100 tablets NDC: 63481-623-757.5 mg/325 mg
Peach, oval-shaped, tablet debossed with “PERCOCET” on one side and “7.5/325” on the other.
Bottles of 100 NDC: 63481-628-707.5 mg/500 mg
Peach, capsule-shaped, tablet debossed with “PERCOCET” on one side and “7.5” on the other.
Bottles of 100 NDC: 63481-621-7010 mg/325 mg
Yellow, capsule-shaped, tablet debossed with “PERCOCET” on one side and “10/325” on the other.
Bottles of 100 NDC: 63481-629-7010 mg/650 mg
Yellow, oval, tablet debossed with “PERCOCET” on one side and “10” on the other.
Bottles of 100 NDC: 63481-622-70Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
DEA Order Form Required.
Manufactured for:
Endo Pharmaceuticals Inc.
Chadds Ford, Pennsylvania 19317PERCOCET® is a Registered Trademark of Endo Pharmaceuticals Inc.
© 2012 Endo Pharmaceuticals
Printed in U.S.A.
8183395 R1
February 2012 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PERCOCET
oxycodone hydrochloride and acetaminophen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63629-3776(NDC: 63481-622) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYCODONE HYDROCHLORIDE (UNII: C1ENJ2TE6C) (OXYCODONE - UNII:CD35PMG570) OXYCODONE HYDROCHLORIDE 10 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (UNII: 68401960MK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color YELLOW Score no score Shape OVAL Size 17mm Flavor Imprint Code PERCOCET;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63629-3776-1 60 in 1 BOTTLE 2 NDC: 63629-3776-2 90 in 1 BOTTLE 3 NDC: 63629-3776-3 120 in 1 BOTTLE 4 NDC: 63629-3776-4 150 in 1 BOTTLE 5 NDC: 63629-3776-5 180 in 1 BOTTLE 6 NDC: 63629-3776-6 25 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040341 07/30/1999 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-3776) , RELABEL(63629-3776)
Trademark Results [PERCOCET]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PERCOCET 73072165 1051682 Live/Registered |
ENDO LABORATORIES, INC. 1975-12-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
