SUDAFED- pseudoephedrine hydrochloride tablet, coated
SUDAFED by
Drug Labeling and Warnings
SUDAFED by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
-
Inactive ingredients
carnauba wax, colloidal silicon dioxide, D&C yellow no. 10 aluminum lake, FD&C red no. 40 aluminum lake, FD&C yellow no. 6 aluminum lake, iron oxide, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, pregelatinized starch, shellac, sodium starch glycolate, talc, titanium dioxide
- Questions or comments?
-
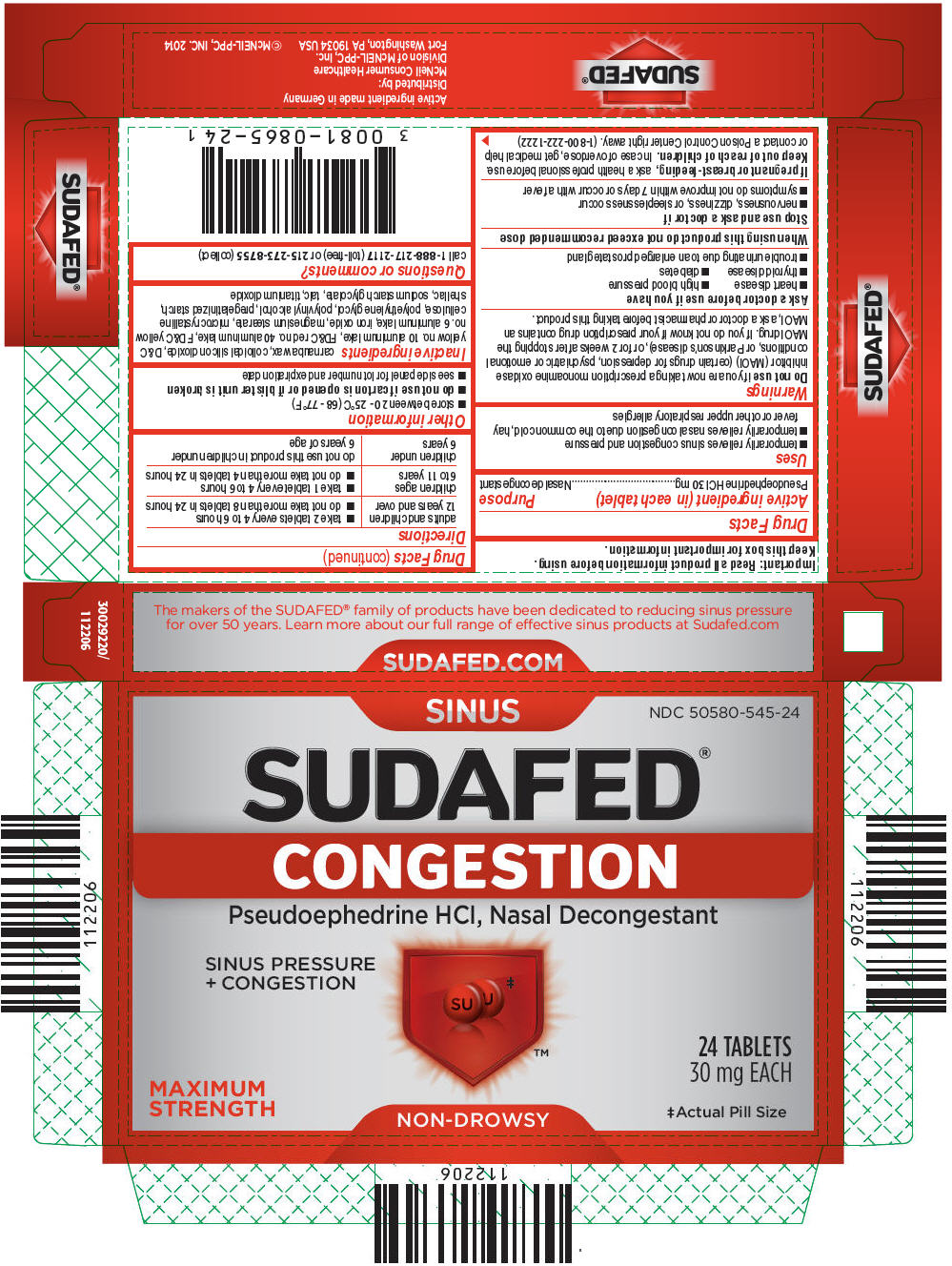
PRINCIPAL DISPLAY PANEL
SINUS
NDC: 50580-545-24
SUDAFED®
CONGESTIONPseudoephedrine HCl, Nasal Decongestant
SINUS PRESSURE
+ CONGESTIONMAXIMUM
STRENGTH24 TABLETS
30 mg EACH‡Actual Pill Size
NON-DROWSY
-
INGREDIENTS AND APPEARANCE
SUDAFED
pseudoephedrine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50580-545 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pseudoephedrine Hydrochloride (UNII: 6V9V2RYJ8N) (Pseudoephedrine - UNII:7CUC9DDI9F) Pseudoephedrine Hydrochloride 30 mg Inactive Ingredients Ingredient Name Strength carnauba wax (UNII: R12CBM0EIZ) silicon dioxide (UNII: ETJ7Z6XBU4) D&C yellow NO. 10 (UNII: 35SW5USQ3G) aluminum oxide (UNII: LMI26O6933) FD&C red NO. 40 (UNII: WZB9127XOA) FD&C yellow NO. 6 (UNII: H77VEI93A8) ferrosoferric oxide (UNII: XM0M87F357) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) polyvinyl alcohol, unspecified (UNII: 532B59J990) shellac (UNII: 46N107B71O) sodium starch glycolate type a potato (UNII: 5856J3G2A2) talc (UNII: 7SEV7J4R1U) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color RED Score no score Shape ROUND Size 7mm Flavor Imprint Code SU Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50580-545-24 3 in 1 CARTON 07/13/2015 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 50580-545-72 3 in 1 PACKAGE 02/29/2016 2 NDC: 50580-545-24 3 in 1 CARTON 2 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 50580-545-48 6 in 1 CARTON 07/13/2015 3 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 10/01/2011 Labeler - Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division (878046358)
Trademark Results [SUDAFED]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUDAFED 98369359 not registered Live/Pending |
KENVUE INC. 2024-01-22 |
 SUDAFED 97338518 not registered Live/Pending |
JOHNSON & JOHNSON 2022-04-05 |
 SUDAFED 78766059 3624735 Dead/Cancelled |
JOHNSON & JOHNSON 2005-12-03 |
 SUDAFED 78158808 not registered Dead/Abandoned |
Warner-Lambert Company 2002-08-28 |
 SUDAFED 77939980 4222270 Live/Registered |
JOHNSON & JOHNSON 2010-02-19 |
 SUDAFED 77939929 4125977 Live/Registered |
JOHNSON & JOHNSON 2010-02-19 |
 SUDAFED 76462993 2741719 Live/Registered |
JOHNSON & JOHNSON 2002-10-28 |
 SUDAFED 73400915 1266826 Live/Registered |
Burroughs Wellcome Co. 1982-10-27 |
 SUDAFED 71623397 0565723 Live/Registered |
BURROUGHS WELLCOME & CO. (U.S.A.) INC. 1952-01-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.