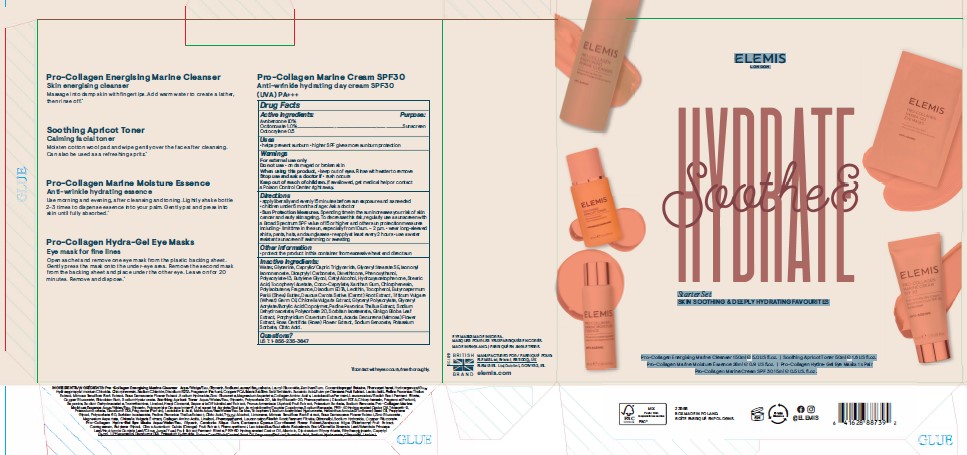
SOOTHE AND HYDRATE STARTER SET- avobenzone, octinoxate, and octocrylene
Elemis Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Questions?
UST: 1-855-235-3647
Inactive Ingredients
Water, Glycerine. Caprylic/Capric Triglyceride, Glyceryl StearateS E. lsononyl
lsononanoate. Dicaprylyt Carbonate, Dimethicone, Phenoxythanol,
Polyacrylate-13. Butylene Glycol. Cetyl Alcohol. Hydroxyacetophenone, Stearic
Acid, Tocopheryl Acetate, Coco-Caprylate. Xanthan Gum, Chlorphenesin,
Polyisobutene, Fragrance, Disodium EDTA, Lecithin, Tocopherol, Butyrospermum
Parkii (Shea) Butter, Daucus Carota Sativa (Carrot) Root Extract. Triticum Vulgare
(Wheat) Germ Oil, Chiarella Vulgaris Extract, Glyceryl Polyacrylate, Glyceryl
Acrylate/Acrylic Acid Copolymer, Padina Pavonica Thallus Extract, Sodium
Dehydroacetate, Polysorbate 20, Sorbitan lsostearate, Ginkgo Biloba Leaf
Extract. Porphyridium Cruentum Extract. Acacia Decurrens (Mimosa) Flower
Extract. Rosa Centifola (Rose) Flower Extract. Sodium Benzoate. Potassium
Sorbate, Citric Acid.
Other information
protect the product in this container from excessive heat and direct sun
Directions
apply liberally and evenly 15 minutes before sun exposure and as needed
children under 6 months of age: Ask a doctor
Sun Protection Measures.S pending time in the sun increases your risk of skin
cancer and early skin ageing, To decrease this risk, regularly use a sunscreen with
a Broad SpectrumS PF value of 15 or higher and other sun protection measures
including· limit time in the sun, especially from 1 0a.m. - 2 p.m. wear long-sleeved
shirts. pants. hats. and sunglasses reapply at least every 2 hours use a water
resistant sunscreen if swimming or sweating
Warnings
For external use only
Do not use on damaged or broken skin
When using this product, keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if rash occurs
Keep oU1 of reach of children. If swallowed, get medical help or contact
a Poison Control Center right away.
Uses
helps prevent sunburn higher SPF gives more sunburn protection
Active Ingredients
:
Avobenzone 1.0%
Octionoxate 1.0%
Octocrylene 0.5
ELEMIS
LONDON
Soothe & Hydrate
Starter Set
SKIN SOOTHING & DEEPLY HYDRATING FAVOURITES
Pro-Collagen Energising Marine Cleanser 150ml e 5.0 US fl.oz. I Soothing Apricot Toner 50ml e 1.6 US fl.oz.
Pro-Collagen Marine Moisture Essence 28ml e 0.9 US fl.oz. I Pro-Collagen Hydra-Gel Eye Masks 1 x Pair
Pro-CollagenMarine Cream SPF 30 15ml e 0.5 US. fl.oz.