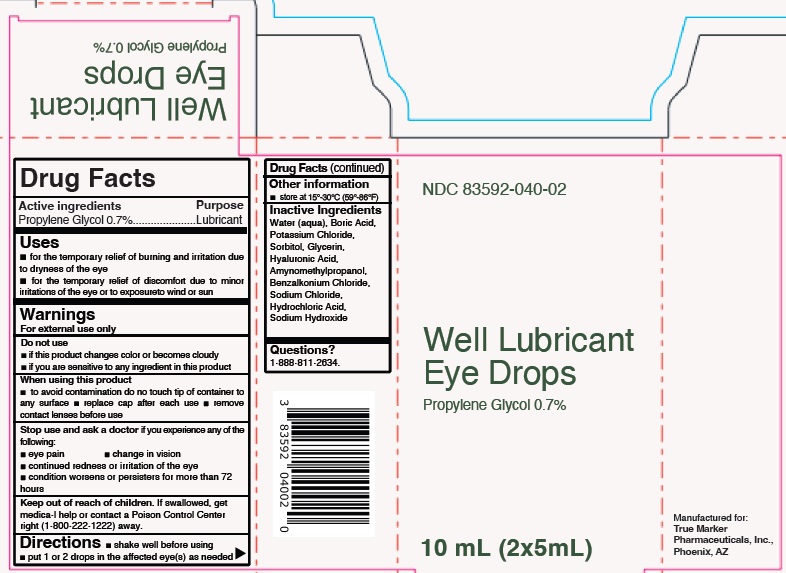
Well Lubricant Eye Drops by True Marker Pharmaceuticals, Inc. Drug Facts
Well Lubricant Eye Drops by
Drug Labeling and Warnings
Well Lubricant Eye Drops by is a Otc medication manufactured, distributed, or labeled by True Marker Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WELL LUBRICANT EYE DROPS- propylene glycol liquid
True Marker Pharmaceuticals, Inc.
----------
Drug Facts
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposureto wind or sun
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- to avoid contamination do no touch tip of container to any surface
- replace cap after each use
- remove contact lenses before use
Stop use and ask a doctor if you experience any of the
following:
- eye pain
- change in vision
- continued redness or irritation of the eye
- condition worsens or persisters for more than 72 hours
Keep out of reach of children. If swallowed, get medica-l help or contact a Poison Control Center right (1-800-222-1222) away.
| WELL LUBRICANT EYE DROPS
propylene glycol liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - True Marker Pharmaceuticals, Inc. (119046582) |
Revised: 11/2024
Document Id: 27eb6e41-bcf1-27f3-e063-6394a90a5ef4
Set id: d29dc2ae-bfae-4bd0-9863-ae463867e1d9
Version: 2
Effective Time: 20241127