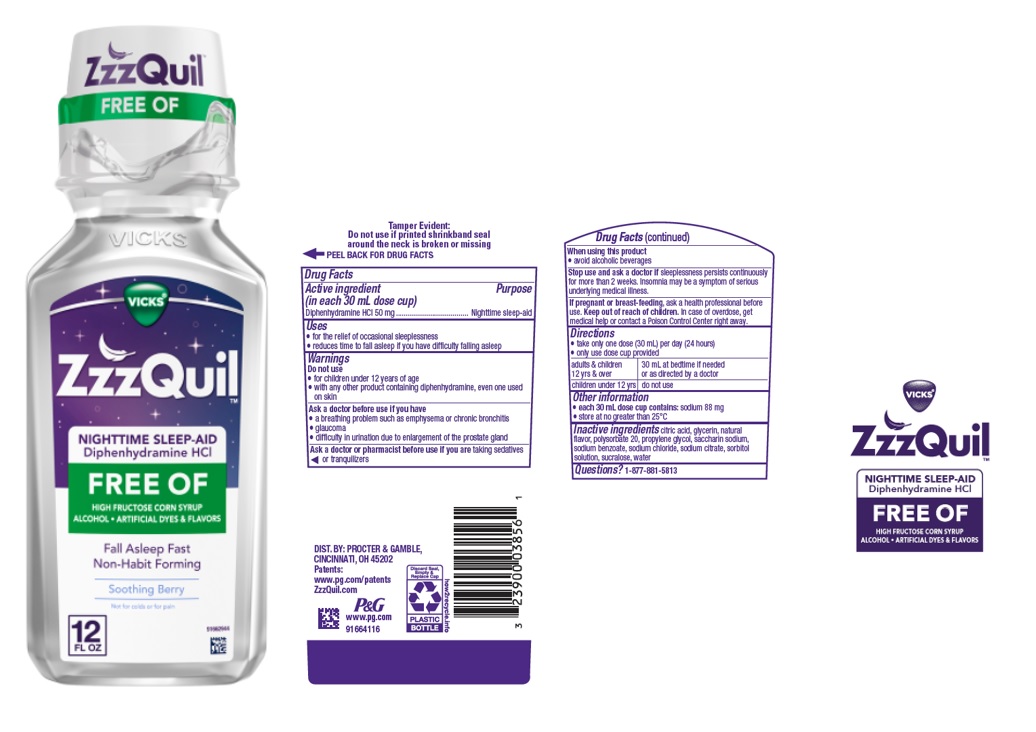
Vicks ZzzQuil™ Nighttime Sleep-Aid Diphenhydramine HCl FREE OF
ZzzQuil by
Drug Labeling and Warnings
ZzzQuil by is a Otc medication manufactured, distributed, or labeled by The Procter & Gamble Manufacturing Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZZZQUIL FREE OF- diphenhydramine hydrochloride solution
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Vicks ZzzQuil™
Nighttime Sleep-Aid
Diphenhydramine HCl
FREE OF
Uses
- for the relief of occasional sleeplessness
- reduces time to fall asleep if you have difficulty falling asleep
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Stop use and ask a doctor
if sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
Directions
- take only one dose (30 mL) per day (24 hours)
- only use dose cup provided
| adults & children 12 yrs & over | 30 mL at bedtime if needed
or as directed by a doctor |
| children under 12 yrs | do not use |
| ZZZQUIL
FREE OF
diphenhydramine hydrochloride solution |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 8/2023
Document Id: 0321c480-e152-421d-e063-6294a90ad687
Set id: d2a50e5a-170f-5530-e053-2a95a90a2f16
Version: 5
Effective Time: 20230817
The Pr
Trademark Results [ZzzQuil]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZZZQUIL 88876867 not registered Live/Pending |
The Procter & Gamble Company 2020-04-17 |
 ZZZQUIL 86183775 not registered Dead/Abandoned |
The Procter & Gamble Company 2014-02-04 |
 ZZZQUIL 85906959 not registered Dead/Abandoned |
The Procter & Gamble Company 2013-04-17 |
 ZZZQUIL 85780147 not registered Dead/Abandoned |
The Procter & Gamble Company 2012-11-15 |
 ZZZQUIL 85002075 4246392 Live/Registered |
The Procter & Gamble Company 2010-03-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.