ALA QUIN- hydrocortisone and iodochlorhydroxyquin cream
Ala Quin by
Drug Labeling and Warnings
Ala Quin by is a Prescription medication manufactured, distributed, or labeled by Crown Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Rx Only
-
Description
Iodochlorhydroxyquin (Clioquinol) is an antifungal agent and a member of a family of drugs called hydroxyquinolines. Chemically, Iodochlorhydroxyquin is 5-chloro-7-iodo-quinolin-8-ol. Its structural formula is:
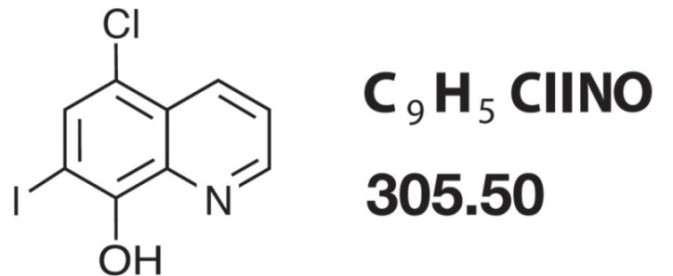
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. Hydrocortisone is a member of this class. Chemically hydrocortisone is pregn-4-ene-3, 20-dione, 11, 17, 21-trihydroxy, (11β)-. Its structural formula is:
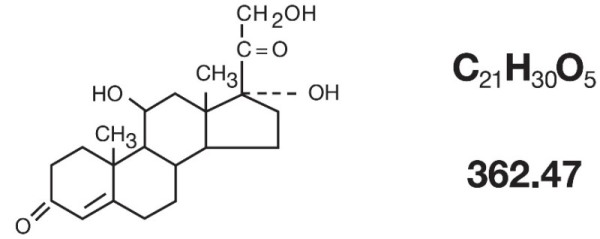
Each gram of ALA-QUIN contains 5 mg Hydrocortisone USP and 30 mg Iodochlorhydroxyquin USP in a cream base consisting of purified water, glycerin, cetyl alcohol, polysorbate 80, stearyl alcohol, sodium lauryl sulfate, cetyl palmitate and sorbic acid.
-
CLINICAL PHARMACOLOGY
Iodochlorhydroxyquin is a broad-spectrum antibacterial and antifungal. Its precise mechanism of action is unknown. Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
-
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of the topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses. (See DOSAGE AND ADMINISTRATION).
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile. -
INDICATIONS AND USAGE
Based on a review of this drug by the National Academy of Sciences-National Research Council and/or other information, FDA has classified the indications as follows:
“Possibly” effective: Contact or atopic dermatitis; impetiginized eczema; nummular eczema; infantile eczema; endogenous chronic infectious dermatitis; stasis dermatitis; pyoderma; nuchal eczema and chronic eczematoid otitis externa; acne urticata; localized or disseminated neurodermatitis; lichen simplex chronicus; anogenital pruritus (vulvae, scroti, ani); folliculitis; bacterial dermatoses; mycotic dermatoses such as tinea (capitis, cruris, corporis, pedis); moniliasis; intertrigo.
Final classification of the less-than-effective indications requires further investigation.
-
CONTRAINDICATIONS
Hypersensitivity to lodochlorhydroxyquin-Hydrocortisone, or any of its ingredients or related compounds: lesions of the eye, tuberculosis of the skin; most viral skin lesions (including herpes simplex, vaccinia, and varicella).
Patients sensitive to chloroxine, iodine, or iodine-containing preparations may also be sensitive to this medication.
-
Precautions
General
Staining of skin and fabrics may occur. Additionally, there are rare reports of discoloration (yellowing) of hair and nails. Iodochlorhydroxyquin-Hydrocortisone may prove irritating to sensitized skin in rare cases. If irritation occurs, discontinue therapy. Check with physician if no improvement within 1 to 2 weeks.
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria in some patients. Signs and symptoms of systemic toxicity, electrolyte imbalance, or adrenal suppression have not been reported with Iodochlorhydroxyquin-Hydrocortisone. Nevertheless, the possibility of suppression of the HPA axis during therapy should be kept in mind, especially when the drug is used under occlusive dressings, for a prolonged period, or for treating extensive cutaneous areas since significant absorption of corticosteroid may occur under these conditions, particularly in children and infants.
Patients receiving a large dose of a potent topical corticosteroid applied to a large surface area or under an occlusive dressing should be evaluated periodically for evidence of HPA axis suppression by using the urinary free cortisol and ACTH stimulation tests. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent corticosteroid.
Recovery of HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids.
Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity (See PRECAUTIONS – Pediatric Use)
If irritation develops, topical corticosteroids should be discontinued and appropriate therapy instituted.
Iodochlorhydroxyquin may be absorbed through the skin and interfere with thyroid function test.
Iodochlorhydroxyquin may cause significant elevation of protein-bound iodine (PBI) or butanol-extractable iodine (BEI) and a decrease in radioactive iodine (RAI) uptake. If such tests are contemplated, wait at least one month between discontinuation of therapy and performance of these tests.
Prolonged use may result in overgrowth of nonsusceptible organisms requiring appropriate therapy. In the presence of systemic infections, appropriate systemic antibiotics should be used.
-
Information for the Patient
Patients using Iodochlorhydroxyquin-Hydrocortisone should receive the following information and instructions:
1. This medication is to be used as directed by the physician.
2. This medication is for external use only. Do not use in or around the eyes. This product is not for ophthalmic use.
3. Patients should be advised not to use this medication for any disorder other than for which it is prescribed.
4. Patients should report any signs of local adverse reactions especially under occlusive dressing.
- Latoratory Tests
- Carcinogenesis, Mutagenesis, and Impairment of Fertility
-
Pregnancy Category C
Although topical steroids have not been reported to have an adverse effect on pregnancy, the safety of their use in pregnant women has not been absolutely established. Use of large amounts or for prolonged periods of time is not recommended since systemic absorption may occur. In laboratory animals, increases in incidence of fetal abnormalities have been associated with exposure of gestating females to topical corticosteroids, in some cases at rather low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topical applied corticosteroids. Topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Therefore, drugs of this class should not be used extensively on pregnant patients in large amounts or for prolonged periods of time.
-
Nursing Mothers
It is not known whether topical administration of this drug could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when this class of drug is administered to a nursing woman.
-
Pediatric Use
Use is not recommended for infants or children up to 2 years of age.
Iodochlorhydroxyquin may produce false-positive ferric chloride test results for phenylketonuria (PKU) if iodochlorhydroxyquin is present in the neonate’s diaper or urine.
Special care must be exercised in using this drug in a pediatric patient. It is recommended that only low-potency topical corticosteroids that are not fluorinated and that have a free 17-hydroxyl group be used in children unless there is a very specific indication for one or the other topical corticosteroids.
As a general rule, pediatric therapy continuing for longer than 2 weeks and consisting of doses in excess of 2 daily applications (with low-potency corticosteroids) should be carefully evaluated by the physician. This is especially important if medication is applied to more than 5-10% of the body surface or if an occlusive dressing is used. A tight-fitting diaper or one covered with plastic pants may constitute an occlusive dressing.
Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
-
ADVERSE REACTIONS
There have been a few reports of rash and hypersensitivity as well as thinning of the skin with easy bruising.
The following local adverse reactions have also been reported with topical corticosteroids and iodochlorhydroxyquin especially under occlusive dressings; burning; itching; irritation; dryness; folliculitis; blistering, peeling, redness, swelling; hypertrichosis; acneiform eruptions; hypopigmentation; perioral dermatitis; allergic contact dermatitis; maceration of the skin, secondary infection; skin atrophy; striae; miliaria or other signs of irritation not present before therapy.
Discontinue therapy if any untoward reaction occurs.
- DOSAGE AND ADMINISTRATION
- PACKAGING AND STORAGE
-
HOW SUPPLIED
ALA-QUIN (Hydrocortisone USP, 0.5% and Iodochlorhydroxyquin USP, 3%) Cream is supplied in
1 ounce tube NDC: 0316-0123-01
80 grams tube NDC: 0316-0123-80
Revised: Oct. 2015
Manufactured and Distributed by: Crown Laboratories, Inc., Johnson City, Tennessee 37604
PRINTED IN USA
P8001.04
-
Ala-Quin 1oz Label
NDC: 0316-0123-01
Rx Only
ALA-QUIN ®
Hydrocortisone 0.5%
Iodochlorhydroxyquin 3% Cream
1 oz (28.4 grams)
WARNING: Keep out of reach of children.
For external use only. Not for ophthalmic use.
See crimp of tube for Lot Number and Expiration Date.
Each gram contains: 5 mg Hydrocortisone USP and 30 mg Iodochlorhydroxyquin USP in a cream base consisting of purified water, glycerin, cetyl alcohol, polysorbate 80, stearyl alcohol, sodium lauryl sulfate, cetyl palmitate and sorbic acid.
Usual Dosage: Apply as a thin film to the affected areas 2 to 4 times daily or as directed by physician. See package insert for full prescribing information.
TO OPEN: Use cap to puncture seal.
IMPORTANT: Do not use if seal has been punctured or is not visible.
Store at 20 o-25 oC (68 o-77 oF) [See USP Controlled Room Temperature].
Protect from freezing.
Manufactured and Distributed by:
Crown Laboratories, Inc.
Johnson City, TN 37604
P6501.03

-
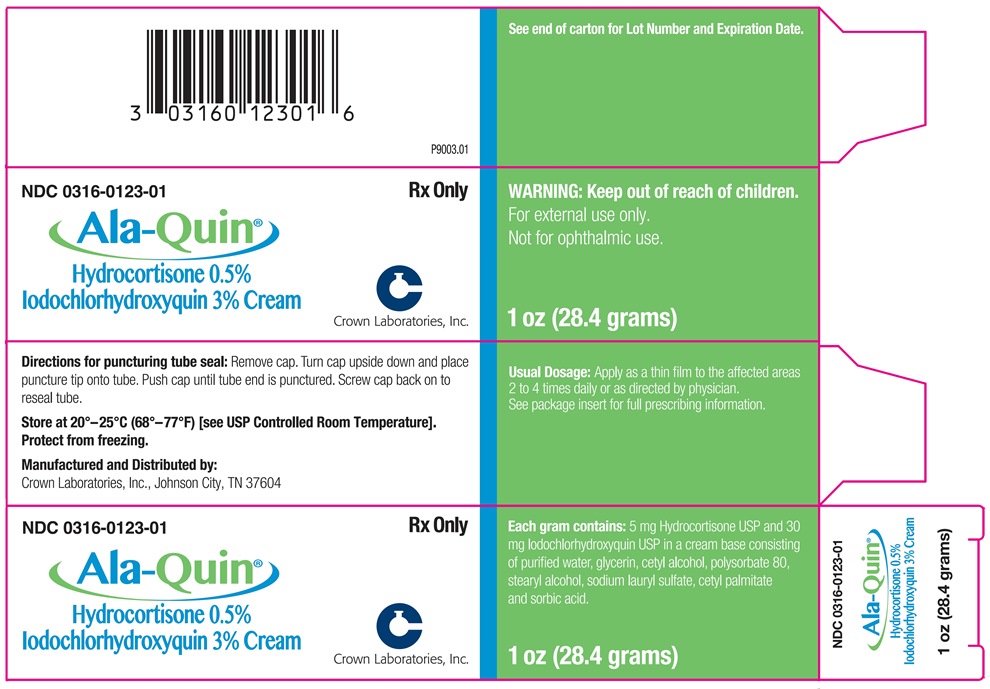
ALA-QUIN 1oz Carton
NDC: 0316-0123-01
Rx Only
ALA-QUIN ®
Hydrocortisone 0.5%
Iodochlorhydroxyquin 3% Cream
1 oz (28.4 grams)
WARNING: Keep out of reach of children.
For external use only.
Not for ophthalmic use.
Usual Dosage: Apply as a thin film to the affected areas 2 to 4 times daily or as directed by physician. See package insert for full prescribing information.
Each gram contains: 5 mg Hydrocortisone USP and 30 mg Iodochlorhydroxyquin USP in a cream base consisting of purified water, glycerin, cetyl alcohol, polysorbate 80, stearyl alcohol, sodium lauryl sulfate, cetyl palmitate and sorbic acid.
Directions for puncturing tube seal: Remove cap. Turn cap upside down and place puncture tip onto tube. Push cap until tube end is punctured. Screw cap back on to reseal tube.
Store at 20 o-25 oC (68 o-77 oF) [See USP Controlled Room Temperature]. Protect from freezing.
See end of carton for Lot Number and Expiration Date.
Manufactured and Distributed by:
Crown Laboratories, Inc.
Johnson City, TN 37604
P9003.01
-
80 Grams Label
NDC: 0316-0123-80
Rx Only
ALA-QUIN ®
Hydrocortisone 0.5%
Iodochlorhydroxyquin 3% Cream
80 grams
WARNING: Keep out of reach of children.
For external use only. Not for ophthalmic use.
See crimp of tube for Lot Number and Expiration Date.
Each gram contains: 5 mg Hydrocortisone USP and 30 mg Iodochlorhydroxyquin USP in a cream base consisting of purified water, glycerin, cetyl alcohol, polysorbate 80, stearyl alcohol, sodium lauryl sulfate, cetyl palmitate and sorbic acid.
Usual Dosage: Apply as a thin film to the affected areas 2 to 4 times daily or as directed by physician. See package insert for full prescribing information.
TO OPEN: Use cap to puncture seal.
IMPORTANT: Do not use if seal has been punctured or is not visible.
Store at 20o-25 oC (68 o-77 oF) [See USP Controlled Room Temperature].
Protect from freezing.
Manufactured and Distributed by:
Crown Laboratories, Inc.
Johnson City, TN 37604
P4763.00

-
Ala Quin 80 grams carton
NDC: 0316-0123-80
Rx Only
ALA-QUIN
Hydrocortisone 0.5%
Iodochlorhydroxyquin 3% Cream
80 grams
WARNING: Keep out of reach of children.
For external use only. Not for ophthalmic use.
Usual Dosage: Apply as a thin film to the affected areas 2 to 4 time daily or as directed by physician. See package insert for full prescribing information.
Each gram contains: 5 mg Hydrocortisone USP and 30 mg Iodochlorhydroxyquin USP in a cream base consisting of purified water, glycerin, cetyl alcohol, polysorbate 80, stearyl alcohol, sodium lauryl sulfate, cetyl palmitate and sorbic acid.
Directions for puncturing tube seal: Remove cap. Turn cap upside down and place puncture tip onto tube. Push cap until tube end is punctured. Screw cap back on to reseal tube.
Store at 20 o-25 oC (68 o-77 oF) [See USP Controlled Room Temperature]. Protect from freezing.
See end of carton for Lot Number and Expiration Date.
Manufactured and Distributed by:
Crown Laboratories, Inc.
Johnson City, TN 37604
P4762.00

-
INGREDIENTS AND APPEARANCE
ALA QUIN
hydrocortisone and iodochlorhydroxyquin creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0316-0123 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 5 mg in 1 g CLIOQUINOL (UNII: 7BHQ856EJ5) (CLIOQUINOL - UNII:7BHQ856EJ5) CLIOQUINOL 30 mg in 1 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CETYL PALMITATE (UNII: 5ZA2S6B08X) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0316-0123-01 1 in 1 CARTON 08/19/1970 11/30/2021 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0316-0123-80 1 in 1 CARTON 11/12/2015 12/31/2020 2 80 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/19/1970 11/30/2021 Labeler - Crown Laboratories (079035945) Registrant - Crown Laboratories (079035945) Establishment Name Address ID/FEI Business Operations Crown Laboratories 079035945 manufacture(0316-0123)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.