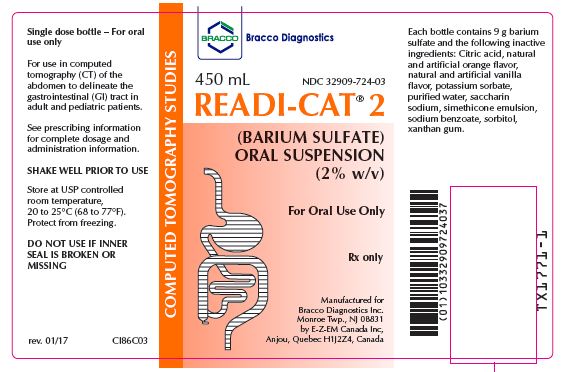
READI-CAT 2- barium sulfate suspension READI-CAT 2 BANANA SMOOTHIE- barium sulfate suspension READI-CAT 2 BERRY SMOOTHIE- barium sulfate suspension READI-CAT 2 MOCHACCINO SMOOTHIE- barium sulfate suspension READI-CAT 2 CREAMY VANILLA SMOOTHIE- barium sulfate suspension
Readi-Cat 2 Creamy Vanilla Smoothie by
Drug Labeling and Warnings
Readi-Cat 2 Creamy Vanilla Smoothie by is a Prescription medication manufactured, distributed, or labeled by E-Z-EM Canada Inc, BRACCO DIAGNOSTICS INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use READI-CAT 2 and READI-CAT 2 SMOOTHIE products safely and effectively. See full prescribing information for
READI-CAT 2 (barium sulfate) oral suspension
READI-CAT 2 SMOOTHIE (barium sulfate) oral suspension
Initial U.S. Approval: 2016RECENT MAJOR CHANGES
Warning and Precautions (5.6) 2/2017 INDICATIONS AND USAGE
READI-CAT 2 and READI-CAT 2 SMOOTHIE are radiographic contrast agents, indicated for use in computed tomography (CT) of the abdomen to delineate the gastrointestinal (GI) tract in adult and pediatric patients (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Oral Suspension: 9 grams barium sulfate (2% w/v) supplied in a single dose HDPE plastic bottle (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Emergency equipment and trained personnel should be immediately available (5.1)
- Intra-abdominal leakage: May occur in conditions such as GI fistula, ulcer, inflammatory bowel disease, appendicitis or diverticulitis, severe stenosis or obstructing lesions of the GI tract (5.2)
- Delayed GI transit and obstruction: Patients should maintain adequate hydration in days following a barium sulfate procedure to avoid obstruction or impaction (5.3)
- Aspiration: Caution is recommended in patients with history of food aspiration and in patients with known swallowing disorders (5.4)
ADVERSE REACTIONS
Common adverse reactions include nausea, vomiting, diarrhea and abdominal cramping (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bracco Diagnostics Inc at 1-800-257-5181 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Intra-abdominal Barium Leakage
5.3 Delayed Gastrointestinal Transit and Obstruction
5.4 Aspiration Pneumonitis
5.5 Systemic Embolization
5.6 Risk with Hereditary Fructose Intolerance
6 ADVERSE REACTIONS
8 USES IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
READI-CAT 2 products are contraindicated in patients:
- with known or suspected perforation of the GI tract
- with known obstruction of the GI tract
- at high risk of GI perforation such as those with a recent prior GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to pelvis
- at high risk of aspiration such as those with prior aspiration, tracheo-esophageal fistula, or obtundation
- known severe hypersensitivity to barium sulfate or any of the excipients of READI-CAT 2 or READI-CAT 2 SMOOTHIES
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Barium sulfate preparations contain a number of excipients, including natural and artificial flavors and may induce serious hypersensitivity reactions. The manifestations include hypotension, bronchospasm and other respiratory impairments, dermal reactions including rashes, urticaria, and itching. A history of bronchial asthma, atopy, or a previous reaction to a contrast agent may increase the risk for hypersensitivity reactions. Emergency equipment and trained personnel should be immediately available for treatment of a hypersensitivity reaction.
5.2 Intra-abdominal Barium Leakage
The use of READI-CAT 2 products is contraindicated in patients at high risk of perforation of the GI tract [see Contraindications (4)]. Administration of READI-CAT 2 products may result in leakage of barium from the GI tract in the presence of conditions such as carcinomas, GI fistula, inflammatory bowel disease, gastric or duodenal ulcer, appendicitis, or diverticulitis, and in patients with a severe stenosis at any level of the GI tract, especially if it is distal to the stomach. The barium leakage has been associated with peritonitis and granuloma formation.
5.3 Delayed Gastrointestinal Transit and Obstruction
Orally administered barium sulfate may accumulate proximal to a constricting lesion of the colon, causing obstruction or impaction with development of baroliths (inspissated barium associated with feces) and may lead to abdominal pain, appendicitis, bowel obstruction, or rarely perforation. Patients with the following conditions are at higher risk for developing obstruction or baroliths: severe stenosis at any level of the GI tract, impaired GI motility, electrolyte imbalance, dehydration, on a low residue diet, taking medications that delay GI motility, and constipation, pediatric patients with cystic fibrosis or Hirschsprung disease, and the elderly [see Use in Specific Populations (8.4, 8.5)]. To reduce the risk of delayed GI transit and obstruction, patients should maintain adequate hydration following a barium sulfate procedure.
5.4 Aspiration Pneumonitis
The use of READI-CAT 2 products is contraindicated in patients at high risk of aspiration [see Contraindications (4)]. Oral administration of barium is associated with aspiration pneumonitis, especially in patients with a history of food aspiration or with compromised swallowing mechanism. Vomiting following oral administration of barium sulfate may lead to aspiration pneumonitis. In patients at risk for aspiration, begin the procedure with a small ingested volume of READI-CAT 2 products. Discontinue administration of READI-CAT 2 products immediately if aspiration is suspected.
5.5 Systemic Embolization
Barium sulfate products may occasionally intravasate into the venous drainage of the large bowel and enter the circulation as a "barium embolus" leading to potentially fatal complications which include systemic and pulmonary embolism, disseminated intravascular coagulation, septicemia and prolonged severe hypotension. Although this complication is exceedingly uncommon after oral administration of barium sulfate suspension, monitor patients for potential intravasation when administering barium sulfate.
5.6 Risk with Hereditary Fructose Intolerance
READI-CAT 2 contains sorbitol which may cause severe reactions if ingested by patients with hereditary fructose intolerance, such as: vomiting, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia, and kidney failure. Before administration of READI-CAT 2 assess patients for a history of hereditary fructose intolerance and avoid use in these patients.
-
6 ADVERSE REACTIONS
The following adverse reactions have been identified from spontaneous reporting or clinical studies of barium sulfate administered orally. Because the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure:
- Nausea, vomiting, diarrhea and abdominal cramping
- Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes
-
8 USES IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
READI-CAT 2 products are not absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure to the drug.8.2 Lactation
Risk Summary
READI-CAT 2 products are not absorbed systemically by the mother following oral administration, and breastfeeding is not expected to result in exposure of the infant to READI-CAT 2.8.4 Pediatric Use
The efficacy of READI-CAT 2 in pediatric patients of all groups is based on successful opacification of the GI tract during radiographic procedures [see Clinical Pharmacology (12.1)].
READI-CAT 2 is contraindicated in pediatric patients with tracheo-esophageal fistula [see Contraindications (4)].
Pediatric patients with a history of asthma or food allergies may be at increased risk for development of hypersensitivity reactions [see Warnings and Precautions (5.1)]. Pediatric patients with cystic fibrosis or Hirschsprung disease should be monitored for bowel obstruction after use [see Warnings and Precautions (5.3)].
8.5 Geriatric Use
Clinical studies of READI-CAT 2 products do not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
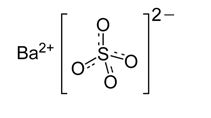
READI-CAT 2 and READI-CAT 2 SMOOTHIE (barium sulfate) are radiographic contrast agents supplied as a suspension (2% w/v) for oral administration. The active ingredient barium sulfate is designated chemically as BaSO4 with a molecular weight of 233.4 g/mol, a density of 4.5 g/cm3, and the following chemical structure:
READI-CAT 2 products contain excipients including: benzoic acid, citric acid, potassium sorbate, saccharin sodium, simethicone emulsion, sodium benzoate, sodium citrate, sorbitol solution, xanthan gum, and purified water.
READI-CAT 2 products also contain natural and artificial flavorings including: banana, blueberry, orange, vanilla, chocolate, and coffee flavors.
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE
AND HANDLING
READI-CAT 2 and READI-CAT 2 SMOOTHIES (barium sulfate) are supplied as suspensions (2 % w/v) in a unit dose in a single-dose HDPE plastic bottle containing 9 grams of barium sulfate in 450 mL.
READI-CAT 2 products are provided in the following flavors as:
READI-CAT 2: (Orange): 24 x 450 mL bottles (NDC: 32909-724-03)
READI-CAT 2 SMOOTHIE (Banana): 24 x 450 mL bottles (NDC: 32909-722-03)
READI-CAT 2 SMOOTHIE (Berry): 24 x 450 mL bottles (NDC: 32909-711-03)
READI-CAT 2 SMOOTHIE (Creamy Vanilla): 24 x 450 mL bottles (NDC: 32909-756-03)
READI-CAT 2 SMOOTHIE (Mochaccino): 24 x 450 mL bottles (NDC: 32909-777-03)Store at USP controlled room temperature, 20 to 25°C (68 to 77° F)
-
17 PATIENT COUNSELING
INFORMATION
After administration advise patients to:
- Maintain adequate hydration
- Seek medical attention for worsening of constipation or slow gastrointestinal passage
- Seek medical attention for any delayed onset of hypersensitivity: rash, urticaria, or respiratory difficulty.
Manufactured by
EZEM Canada Inc
Anjou (Quebec) Canada H1J 2Z4 - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
READI-CAT 2
barium sulfate suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 32909-724 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Barium Sulfate (UNII: 25BB7EKE2E) (Barium Sulfate - UNII:25BB7EKE2E) Barium Sulfate 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) dimethicone 350 (UNII: 2Y53S6ATLU) dimethicone 1000 (UNII: MCU2324216) potassium sorbate (UNII: 1VPU26JZZ4) silicon dioxide (UNII: ETJ7Z6XBU4) sodium benzoate (UNII: OJ245FE5EU) sorbitol (UNII: 506T60A25R) water (UNII: 059QF0KO0R) xanthan gum (UNII: TTV12P4NEE) saccharin sodium (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 32909-724-03 24 in 1 CARTON 03/27/2017 1 450 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208143 03/27/2017 READI-CAT 2 BANANA SMOOTHIE
barium sulfate suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 32909-722 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Barium Sulfate (UNII: 25BB7EKE2E) (Barium Sulfate - UNII:25BB7EKE2E) Barium Sulfate 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) benzoic acid (UNII: 8SKN0B0MIM) dimethicone 350 (UNII: 2Y53S6ATLU) dimethicone 1000 (UNII: MCU2324216) potassium sorbate (UNII: 1VPU26JZZ4) silicon dioxide (UNII: ETJ7Z6XBU4) sodium benzoate (UNII: OJ245FE5EU) trisodium citrate dihydrate (UNII: B22547B95K) sorbitol (UNII: 506T60A25R) water (UNII: 059QF0KO0R) xanthan gum (UNII: TTV12P4NEE) saccharin sodium (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor BANANA, VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 32909-722-03 24 in 1 CARTON 03/27/2017 1 450 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208143 03/27/2017 READI-CAT 2 BERRY SMOOTHIE
barium sulfate suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 32909-711 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Barium Sulfate (UNII: 25BB7EKE2E) (Barium Sulfate - UNII:25BB7EKE2E) Barium Sulfate 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) benzoic acid (UNII: 8SKN0B0MIM) dimethicone 350 (UNII: 2Y53S6ATLU) dimethicone 1000 (UNII: MCU2324216) potassium sorbate (UNII: 1VPU26JZZ4) silicon dioxide (UNII: ETJ7Z6XBU4) sodium benzoate (UNII: OJ245FE5EU) trisodium citrate dihydrate (UNII: B22547B95K) sorbitol (UNII: 506T60A25R) water (UNII: 059QF0KO0R) xanthan gum (UNII: TTV12P4NEE) saccharin sodium (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor BLUEBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 32909-711-03 24 in 1 CARTON 03/27/2017 1 450 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208143 03/27/2017 READI-CAT 2 MOCHACCINO SMOOTHIE
barium sulfate suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 32909-777 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Barium Sulfate (UNII: 25BB7EKE2E) (Barium Sulfate - UNII:25BB7EKE2E) Barium Sulfate 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) benzoic acid (UNII: 8SKN0B0MIM) dimethicone 350 (UNII: 2Y53S6ATLU) dimethicone 1000 (UNII: MCU2324216) potassium sorbate (UNII: 1VPU26JZZ4) silicon dioxide (UNII: ETJ7Z6XBU4) sodium benzoate (UNII: OJ245FE5EU) trisodium citrate dihydrate (UNII: B22547B95K) sorbitol (UNII: 506T60A25R) water (UNII: 059QF0KO0R) xanthan gum (UNII: TTV12P4NEE) saccharin sodium (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor COFFEE, CHOCOLATE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 32909-777-03 24 in 1 CARTON 03/27/2017 1 450 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208143 03/27/2017 READI-CAT 2 CREAMY VANILLA SMOOTHIE
barium sulfate suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 32909-756 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Barium Sulfate (UNII: 25BB7EKE2E) (Barium Sulfate - UNII:25BB7EKE2E) Barium Sulfate 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) benzoic acid (UNII: 8SKN0B0MIM) dimethicone 350 (UNII: 2Y53S6ATLU) dimethicone 1000 (UNII: MCU2324216) potassium sorbate (UNII: 1VPU26JZZ4) silicon dioxide (UNII: ETJ7Z6XBU4) sodium benzoate (UNII: OJ245FE5EU) trisodium citrate dihydrate (UNII: B22547B95K) sorbitol (UNII: 506T60A25R) water (UNII: 059QF0KO0R) xanthan gum (UNII: TTV12P4NEE) saccharin sodium (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 32909-756-03 24 in 1 CARTON 03/27/2017 1 450 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208143 03/27/2017 Labeler - E-Z-EM Canada Inc (204211163) Registrant - BRACCO DIAGNOSTICS INC (849234661) Establishment Name Address ID/FEI Business Operations E-Z-EM Canada Inc 204211163 ANALYSIS(32909-777, 32909-711, 32909-722, 32909-756, 32909-724) , MANUFACTURE(32909-711, 32909-777, 32909-722, 32909-756, 32909-724)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.