NEMEX 2 NON-STERILE- pyrantel pamoate suspension
Nemex 2 by
Drug Labeling and Warnings
Nemex 2 by is a Animal medication manufactured, distributed, or labeled by Zoetis, Megafine Pharma (P) Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Nemex-2 is a suspension of pyrantel pamoate in a palatable caramel-flavored vehicle. Each mL contains 4.54 mg of pyrantel base as pyrantel pamoate.
Pyrantel pamoate is a compound belonging to a family classified chemically as tetrahydropyrimidines. It is a yellow, water-insoluble crystalline salt of the tetrahydropyrimidine base and pamoic acid containing 34.7% base activity. The chemical structure and name are given below:
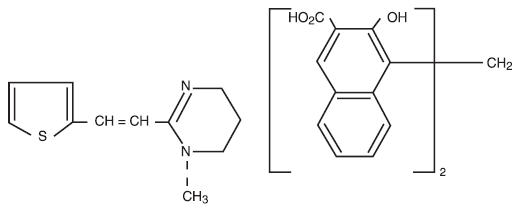
E)-1,4,5,6-Tetrahydro-1-methyl-2-[2-(2-thienyl) vinyl] pyrimidine 4,4' methylenebis [3-hydroxy-2-naphthoate] (1:1)
-
INDICATIONS AND USAGE
Nemex-2 suspension is a highly palatable formulation intended as a single treatment for the removal of large roundworms (Toxocara canis and Toxascaris leonina) and hookworms (Ancylostoma caninum and Uncinaria stenocephala) in dogs and puppies. The presence of these parasites should be confirmed by laboratory fecal exam. Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
Nemex-2 suspension may also be used to prevent reinfestation of T. canis in puppies and adult dogs and in lactating bitches after whelping.
- PRECAUTIONS
-
DOSAGE AND ADMINISTRATION
Administer 1 teaspoon (5 mL) for each 10 lb of body weight. It is not necessary to withhold food prior to or after treatment. Dogs usually find this dewormer very palatable and will lick the dose from the bowl willingly. If there is reluctance to accept the dose, mix in a small quantity of dog food to encourage consumption. It is recommended that dogs maintained under conditions of constant exposure to worm infestation should have a follow-up fecal exam within 2–4 weeks after treatment.
For maximum control and prevention of reinfestation, it is recommended that puppies be treated at 2, 3, 4, 6, 8, and 10 weeks of age. Lactating bitches should be treated 2–3 weeks after whelping. Adult dogs kept in heavily contaminated quarters may be treated at monthly intervals to prevent T. canis reinfestation.
-
SAFETY AND EFFICACY STUDIES
Critical (worm count) studies in dogs demonstrated that Nemex-2 at the recommended dosage is highly efficacious against T. leonina (99%), T. canis (85%), A. caninum (97%), and U. stenocephala (94%).
One of the most outstanding and significant features of Nemex-2 is its wide margin of therapeutic safety in dogs. The acute oral LD50 of pyrantel pamoate administered in gelatin capsules to female and male dogs is greater than 314 mg base per lb of body weight, which indicates a therapeutic index in excess of 138 times the recommended dosage. In subacute and chronic studies, no significant morphological abnormalities could be attributed to Nemex-2 when administered to dogs at daily dose rates up to 94 mg base per lb of body weight (40x) for periods of 19, 30, and 90 days.
Clinical studies conducted in a wide variety of geographic locations using more than 40 different breeds of dogs showed no drug-induced toxic effects. Included in these studies were nursing pups, weaned pups, adults, pregnant bitches, and males at stud. Additional data have demonstrated the safe use of Nemex-2 in dogs having heartworm infestations and/or receiving medication for heartworms, dogs exposed to organophosphate flea collars or flea/tick dip treatments, and dogs undergoing concurrent treatment or medication at the time of worming such as immunization and antibacterial treatment.
- RECOMMENDED STORAGE
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
NEMEX 2 NON-STERILE
pyrantel pamoate suspensionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 54771-2934 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pyrantel pamoate (UNII: 81BK194Z5M) (pyrantel - UNII:4QIH0N49E7) pyrantel 4.54 mg in 1 mL Product Characteristics Color Score Shape Size Flavor CARAMEL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-2934-1 60 mL in 1 BOTTLE 2 NDC: 54771-2934-2 473 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA100237 06/22/1979 Labeler - Zoetis (828851555) Establishment Name Address ID/FEI Business Operations Megafine Pharma (P) Limited 925627080 API MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

