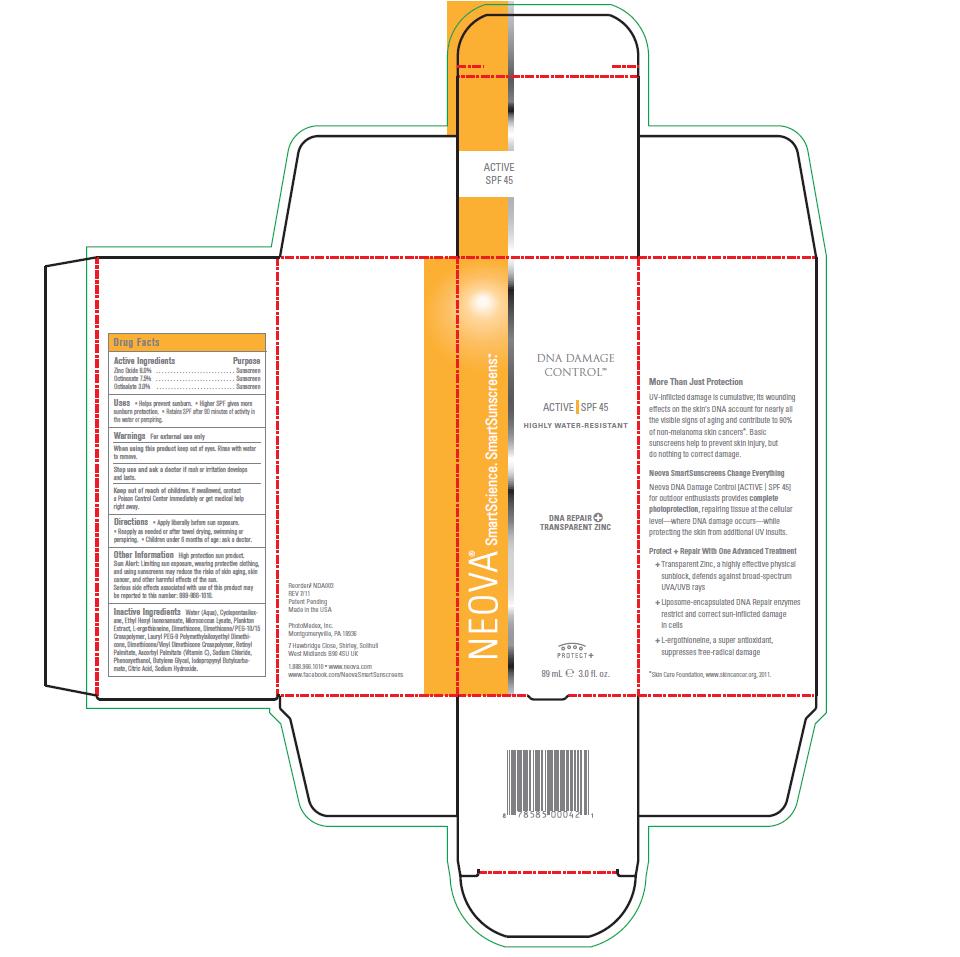
NEOVA DNA DAMAGE CONTROL - ACTIVE SPF 45- zinc oxide, octinoxate, octisalate emulsion
Neova DNA Damage Control - Active by
Drug Labeling and Warnings
Neova DNA Damage Control - Active by is a Otc medication manufactured, distributed, or labeled by PhotoMedex, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
-
Other Information
High protection sun product. Sun Alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun. Serious side effects associated with use of this product may be reported to this number: 888-966-1010.
-
Inactive Ingredients
Water (Aqua), Cyclopentasiloxane,
Ethyl Hexyl Isononanoate, Micrococcus Lysate, Plankton
Extract, L-ergothioneine, Dimethicone, Dimethicone/PEG-10/15
Crosspolymer, Lauryl PEG-9 Polymethylsiloxyethyl Dimethicone,
Dimethicone/Vinyl Dimethicone Crosspolymer, Retinyl
Palmitate, Ascorbyl Palmitate (Vitamin C), Sodium Chloride,
Phenoxyethanol, Butylene Glycol, Iodopropynyl Butylcarbamate,
Citric Acid, Sodium Hydroxide. - Image of Box and Label
-
INGREDIENTS AND APPEARANCE
NEOVA DNA DAMAGE CONTROL - ACTIVE SPF 45
zinc oxide, octinoxate, octisalate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62362-129 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 8 mL in 100 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 7.5 mL in 100 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 3 mL in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Ethylhexyl Isononanoate (UNII: I6KB4GE3K4) Micrococcus Luteus (UNII: LV6L29Z6AX) Ergothioneine (UNII: BDZ3DQM98W) Dimethicone (UNII: 92RU3N3Y1O) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Ascorbyl Palmitate (UNII: QN83US2B0N) Sodium Chloride (UNII: 451W47IQ8X) Phenoxyethanol (UNII: HIE492ZZ3T) Butylene Glycol (UNII: 3XUS85K0RA) Iodopropynyl Butylcarbamate (UNII: 603P14DHEB) Citric Acid (UNII: 2968PHW8QP) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62362-129-01 1 in 1 BOX 1 NDC: 62362-129-89 89 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/05/2012 Labeler - PhotoMedex, Inc. (054503875) Establishment Name Address ID/FEI Business Operations PhotoMedex, Inc. 054503875 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.