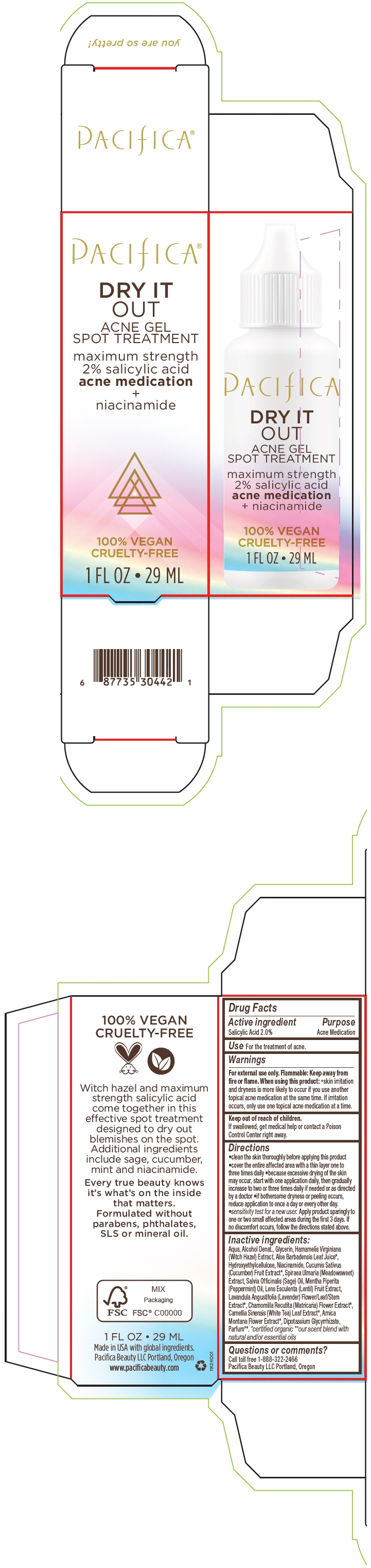
DRY IT OUT ACNE SPOT TREATMENT- salicylic acid gel
DRY IT OUT ACNE SPOT TREATMENT by
Drug Labeling and Warnings
DRY IT OUT ACNE SPOT TREATMENT by is a Otc medication manufactured, distributed, or labeled by Pacifica Beauty LLC., Sun Deep Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- sensitivity test for a new user. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
-
Inactive ingredients
Aqua, Alcohol Denat., Glycerin, Hamamelis Virginiana (Witch Hazel) Extract, Aloe Barbadensis Leaf Juice1, Hydroxyethylcellulose, Niacinamide, Cucumis Sativus (Cucumber) Fruit Extract1, Spiraea Ulmaria (Meadowsweet) Extract, Salvia Officinalis (Sage) Oil, Mentha Piperita (Peppermint) Oil, Lens Esculenta (Lentil) Fruit Extract, Lavandula Angustifolia (Lavender) Flower/Leaf/Stem Extract1, Chamomilla Recutita (Matricaria) Flower Extract1, Camellia Sinensis (White Tea) Leaf Extract1, Arnica Montana Flower Extract1, Dipotassium Glycyrrhizate, Parfum2.
- 1 certified organic
- 2 our scent blend with natural and/or essential oils
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 29 ML Tube Carton
-
INGREDIENTS AND APPEARANCE
DRY IT OUT ACNE SPOT TREATMENT
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61197-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alcohol (UNII: 3K9958V90M) Glycerin (UNII: PDC6A3C0OX) Hamamelis Virginiana Top (UNII: UDA30A2JJY) Aloe Vera Leaf (UNII: ZY81Z83H0X) Niacinamide (UNII: 25X51I8RD4) Cucumber (UNII: YY7C30VXJT) Sage Oil (UNII: U27K0H1H2O) Peppermint Oil (UNII: AV092KU4JH) Lens Culinaris Fruit (UNII: ZYZ076G9JH) Lavandula Angustifolia Subsp. Angustifolia Flowering Top (UNII: 9YT4B71U8P) Chamomile (UNII: FGL3685T2X) White Tea (UNII: O0M3396E09) Arnica Montana Flower (UNII: OZ0E5Y15PZ) Glycyrrhizinate Dipotassium (UNII: CA2Y0FE3FX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61197-102-00 1 in 1 CARTON 02/01/2019 1 29 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 02/01/2019 Labeler - Pacifica Beauty LLC. (058549421) Establishment Name Address ID/FEI Business Operations Sun Deep Inc. 189788201 MANUFACTURE(61197-102)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.