ALLERGY RELIEF- diphenhydramine hydrochloride tablet
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by Health Pharma USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
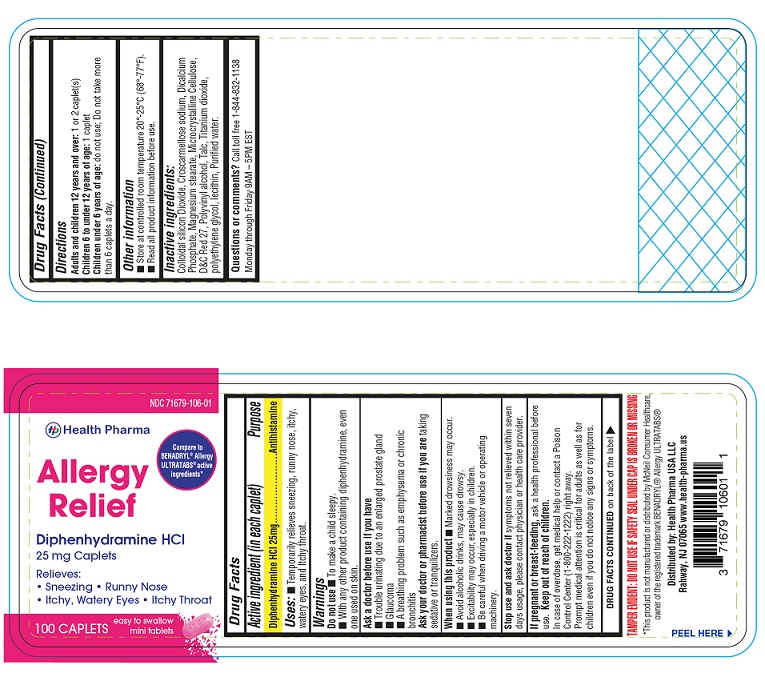
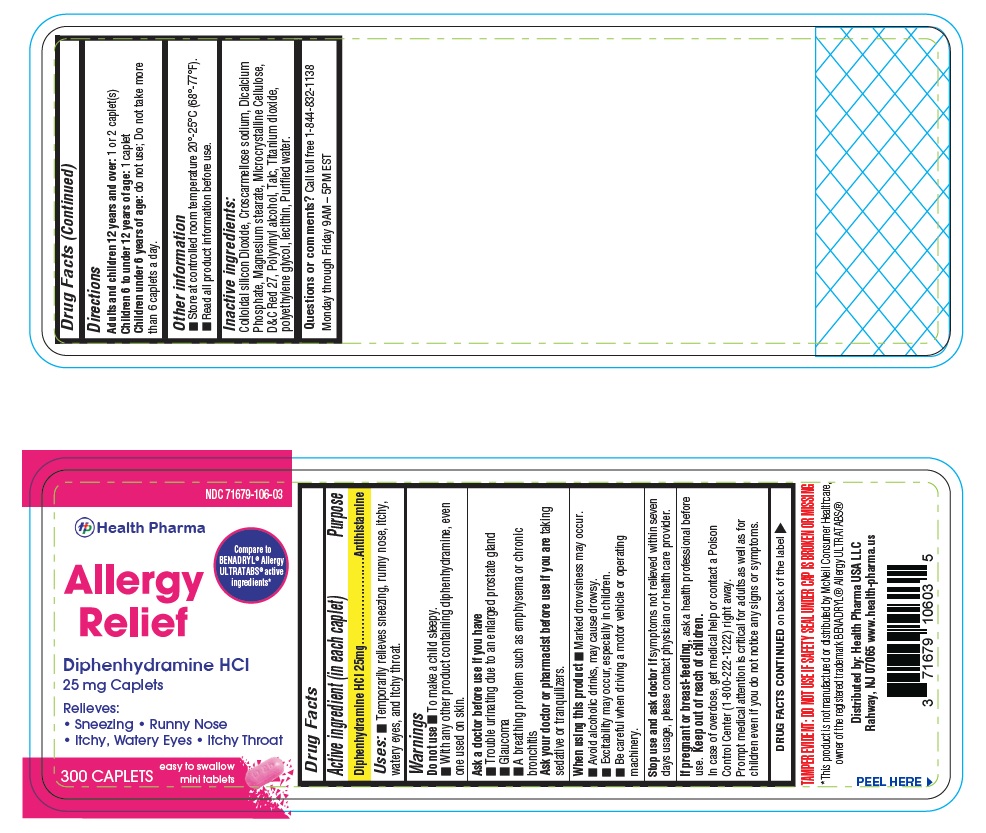
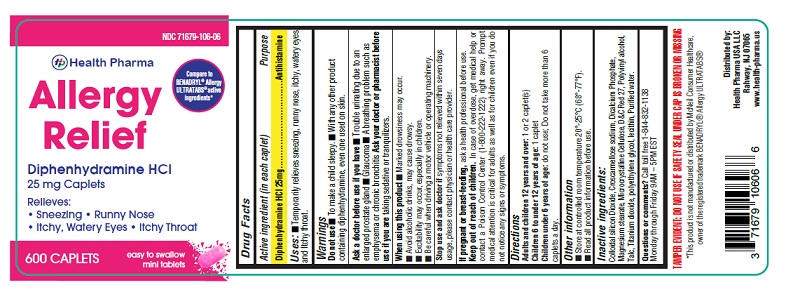
- Active ingredient (in each caplet)
- Purpose
- Uses:
-
Warnings
Do not use
- To make a child sleepy
- With any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- Trouble urinating due to an enlarged prostate gland
- Glaucoma
- A breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
When using this product
- Marked drowsiness may occur.
- Avoid alcoholic drinks, may cause drowsy.
- Excitability may occur, especially in children.
- Be careful when driving a motor vehicle or operating machinery.
Stop use and ask doctor ifsymptoms not relieved within seven days usage, please contact physician or health care provider.
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients:
- Questions or comments?
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-100 count
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-300 count
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-600 count
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-1000 count
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71679-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) D&C RED NO. 27 (UNII: 2LRS185U6K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color pink Score no score Shape CAPSULE Size 11mm Flavor Imprint Code D25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71679-106-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/24/2025 2 NDC: 71679-106-03 300 in 1 BOTTLE; Type 0: Not a Combination Product 06/24/2025 3 NDC: 71679-106-06 600 in 1 BOTTLE; Type 0: Not a Combination Product 06/24/2025 4 NDC: 71679-106-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/24/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/24/2025 Labeler - Health Pharma USA LLC (080804485) Registrant - Health Pharma USA LLC (080804485) Establishment Name Address ID/FEI Business Operations ELYSIUM PHARMACEUTICALS LIMITED 915664486 manufacture(71679-106) Establishment Name Address ID/FEI Business Operations HHH PHARMA USA LLC 062788820 pack(71679-106)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.