ARIXTRA- fondaparinux sodium injection, solution
Arixtra by
Drug Labeling and Warnings
Arixtra by is a Prescription medication manufactured, distributed, or labeled by Mylan Institutional LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ARIXTRA safely and effectively. See full prescribing information for ARIXTRA.
ARIXTRA (fondaparinux sodium injection) solution for subcutaneous injection
Initial U.S. Approval: 2001WARNING: SPINAL/EPIDURAL HEMATOMAS
See full prescribing information for complete boxed warning.
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH), heparinoids, or fondaparinux sodium and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- use of indwelling epidural catheters
- concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, or other anticoagulants
- a history of traumatic or repeated epidural or spinal puncture
- a history of spinal deformity or spinal surgery
Monitor patients frequently for signs and symptoms of neurologic impairment. If neurologic compromise is noted, urgent treatment is necessary.
Consider the benefit and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions (5.1) and Drug Interactions (7)].
INDICATIONS AND USAGE
ARIXTRA is a Factor Xa inhibitor (anticoagulant) indicated for:
- Prophylaxis of deep vein thrombosis (DVT) in patients undergoing hip fracture surgery (including extended prophylaxis), hip replacement surgery, knee replacement surgery, or abdominal surgery. (1.1)
- Treatment of DVT or acute pulmonary embolism (PE) when administered in conjunction with warfarin. (1.2, 1.3)
DOSAGE AND ADMINISTRATION
- Prophylaxis of deep vein thrombosis: ARIXTRA 2.5 mg subcutaneously once daily after hemostasis has been established. The initial dose should be given no earlier than 6 to 8 hours after surgery and continued for 5 to 9 days. For patients undergoing hip fracture surgery, extended prophylaxis up to 24 additional days is recommended. (2.1, 2.2)
- Treatment of deep vein thrombosis and pulmonary embolism: ARIXTRA 5 mg (body weight <50 kg), 7.5 mg (50 to 100 kg), or 10 mg (>100 kg) subcutaneously once daily. Treatment should continue for at least 5 days until INR 2 to 3 achieved with warfarin sodium. (2.3)
Do not use as intramuscular injection. For subcutaneous use, do not mix with other injections or infusions.
DOSAGE FORMS AND STRENGTHS
Single-dose, prefilled syringes containing 2.5 mg, 5 mg, 7.5 mg, or 10 mg of fondaparinux sodium. (3)
CONTRAINDICATIONS
ARIXTRA is contraindicated in the following conditions: (4)
- Severe renal impairment (creatinine clearance <30 mL/min) in prophylaxis or treatment of venous thromboembolism.
- Active major bleeding.
- Bacterial endocarditis.
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of fondaparinux sodium.
- Body weight <50 kg (venous thromboembolism prophylaxis only).
- History of serious hypersensitivity reaction (e.g., angioedema, anaphylactoid/anaphylactic reactions) to ARIXTRA.
WARNINGS AND PRECAUTIONS
- Spinal or epidural hematomas, which may result in long-term or permanent paralysis, can occur. (5.1)
- Patients taking ARIXTRA with risk factors for bleeding are at increased risk of hemorrhage. (5.2)
- Bleeding risk is increased in renal impairment and in patients with low body weight <50 kg. (5.3, 5.4)
- Thrombocytopenia can occur with administration of ARIXTRA. (5.5)
- Periodic routine complete blood counts (including platelet counts), serum creatinine level, and stool occult blood tests are recommended. (5.6)
- The packaging (needle guard) contains dry natural rubber and may cause allergic reactions in latex sensitive individuals. (5.7)
ADVERSE REACTIONS
The most serious adverse reactions associated with the use of ARIXTRA are bleeding complications. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Discontinue agents that may enhance the risk of hemorrhage prior to initiation of therapy with ARIXTRA unless essential. If co-administration is necessary, monitor patients closely for hemorrhage. (7)
USE IN SPECIFIC POPULATIONS
- Safety and effectiveness of ARIXTRA in pediatric patients have not been established. Because the risk for bleeding during treatment with ARIXTRA is increased in adults who weigh <50 kg, bleeding may be a particular safety concern for use of ARIXTRA in the pediatric population. (4, 5.4)
- Because elderly patients are more likely to have reduced renal function, ARIXTRA should be used with caution in these patients. (8.5)
- The risk of bleeding is increased with reduced renal or hepatic function. (8.6, 8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SPINAL/EPIDURAL HEMATOMAS
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Deep Vein Thrombosis
1.2 Treatment of Acute Deep Vein Thrombosis
1.3 Treatment of Acute Pulmonary Embolism
2 DOSAGE AND ADMINISTRATION
2.1 Deep Vein Thrombosis Prophylaxis Following Hip Fracture, Hip Replacement, and Knee Replacement Surgery
2.2 Deep Vein Thrombosis Prophylaxis Following Abdominal Surgery
2.3 Deep Vein Thrombosis and Pulmonary Embolism Treatment
2.4 Hepatic Impairment
2.5 Instructions for Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neuraxial Anesthesia and Post-operative Indwelling Epidural Catheter Use
5.2 Hemorrhage
5.3 Renal Impairment and Bleeding Risk
5.4 Body Weight <50 kg and Bleeding Risk
5.5 Thrombocytopenia
5.6 Monitoring: Laboratory Tests
5.7 Latex
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Local Reactions
6.3 Elevations of Serum Aminotransferases
6.4 Other Adverse Reactions
6.5 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Special Populations
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery
14.2 Extended Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery
14.3 Prophylaxis of Thromboembolic Events Following Hip Replacement Surgery
14.4 Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery
14.5 Prophylaxis of Thromboembolic Events Following Abdominal Surgery in Patients at Risk for Thromboembolic Complications
14.6 Treatment of Deep Vein Thrombosis
14.7 Treatment of Pulmonary Embolism
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Patient Advice
17.2 FDA-Approved Patient Labeling
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SPINAL/EPIDURAL HEMATOMAS
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH), heparinoids, or fondaparinux sodium and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- use of indwelling epidural catheters
- concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, or other anticoagulants
- a history of traumatic or repeated epidural or spinal puncture
- a history of spinal deformity or spinal surgery
- Optimal timing between the administration of ARIXTRA and neuraxial procedures is not known.
Monitor patients frequently for signs and symptoms of neurologic impairment. If neurologic compromise is noted, urgent treatment is necessary.
Consider the benefit and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions (5.1) and Drug Interactions (7)].
-
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Deep Vein Thrombosis
ARIXTRA® is indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE):
- in patients undergoing hip fracture surgery, including extended prophylaxis;
- in patients undergoing hip replacement surgery;
- in patients undergoing knee replacement surgery;
- in patients undergoing abdominal surgery who are at risk for thromboembolic complications.
-
2 DOSAGE AND ADMINISTRATION
Do not mix other medications or solutions with ARIXTRA. Administer ARIXTRA only subcutaneously. Discard unused portion.
2.1 Deep Vein Thrombosis Prophylaxis Following Hip Fracture, Hip Replacement, and Knee Replacement Surgery
In patients undergoing hip fracture, hip replacement, or knee replacement surgery, the recommended dose of ARIXTRA is 2.5 mg administered by subcutaneous injection once daily after hemostasis has been established. Administer the initial dose no earlier than 6 to 8 hours after surgery. Administration of ARIXTRA earlier than 6 hours after surgery increases the risk of major bleeding. The usual duration of therapy is 5 to 9 days; up to 11 days of therapy was administered in clinical trials.
In patients undergoing hip fracture surgery, an extended prophylaxis course of up to 24 additional days is recommended. In patients undergoing hip fracture surgery, a total of 32 days (peri-operative and extended prophylaxis) was administered in clinical trials [see Warnings and Precautions (5.6), Adverse Reactions (6), and Clinical Studies (14)].
2.2 Deep Vein Thrombosis Prophylaxis Following Abdominal Surgery
In patients undergoing abdominal surgery, the recommended dose of ARIXTRA is 2.5 mg administered by subcutaneous injection once daily after hemostasis has been established. Administer the initial dose no earlier than 6 to 8 hours after surgery. Administration of ARIXTRA earlier than 6 hours after surgery increases the risk of major bleeding. The usual duration of administration is 5 to 9 days, and up to 10 days of ARIXTRA was administered in clinical trials.
2.3 Deep Vein Thrombosis and Pulmonary Embolism Treatment
In patients with acute symptomatic DVT and in patients with acute symptomatic PE, the recommended dose of ARIXTRA is 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), or 10 mg (body weight >100 kg) by subcutaneous injection once daily (ARIXTRA treatment regimen). Initiate concomitant treatment with warfarin sodium as soon as possible, usually within 72 hours. Continue treatment with ARIXTRA for at least 5 days and until a therapeutic oral anticoagulant effect is established (INR 2 to 3). The usual duration of administration of ARIXTRA is 5 to 9 days; up to 26 days of ARIXTRA injection was administered in clinical trials [see Warnings and Precautions (5.6), Adverse Reactions (6), and Clinical Studies (14)].
2.4 Hepatic Impairment
No dose adjustment is recommended in patients with mild to moderate hepatic impairment, based upon single-dose pharmacokinetic data. Pharmacokinetic data are not available for patients with severe hepatic impairment. Patients with hepatic impairment may be particularly vulnerable to bleeding during ARIXTRA therapy. Observe these patients closely for signs and symptoms of bleeding [see Clinical Pharmacology (12.4)].
2.5 Instructions for Use
ARIXTRA Injection is provided in a single-dose, prefilled syringe affixed with an automatic needle protection system. ARIXTRA is administered by subcutaneous injection. It must not be administered by intramuscular injection. ARIXTRA is intended for use under a physician’s guidance. Patients may self-inject only if their physician determines that it is appropriate and the patients are trained in subcutaneous injection techniques.
Prior to administration, visually inspect ARIXTRA to ensure the solution is clear and free of particulate matter.
To avoid the loss of drug when using the prefilled syringe, do not expel the air bubble from the syringe before the injection. Administration should be made in the fatty tissue, alternating injection sites (e.g., between the left and right anterolateral or the left and right posterolateral abdominal wall).
To administer ARIXTRA:
- 1. Wipe the surface of the injection site with an alcohol swab.
- 2. Hold the syringe with either hand and use your other hand to twist the rigid needle guard (covers the needle) counter-clockwise. Pull the rigid needle guard straight off the needle (Figure 1). Discard the needle guard.
- 3. Do not try to remove the air bubbles from the syringe before giving the injection.
- 4. Pinch a fold of skin at the injection site between your thumb and forefinger and hold it throughout the injection.
- 5. Hold the syringe with your thumb on the top pad of the plunger rod and your next 2 fingers on the finger grips on the syringe barrel. Pay attention to avoid sticking yourself with the exposed needle (Figure 2).
- 6. Insert the full length of the syringe needle perpendicularly into the skin fold held between the thumb and forefinger (Figure 3).
- 7. Push the plunger rod firmly with your thumb as far as it will go. This will ensure you have injected all the contents of the syringe (Figure 4).
- 8. When you have injected all the contents of the syringe, the plunger should be released. The plunger will then rise automatically while the needle withdraws from the skin and retracts into the security sleeve. Discard the syringe into the sharps container.
- 9.
You will know that the syringe has worked when:
- The needle is pulled back into the security sleeve and the white safety indicator appears above the upper body.
- You may also hear or feel a soft click when the plunger rod is released fully.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ARIXTRA is contraindicated in the following conditions:
- Severe renal impairment (creatinine clearance [CrCl] <30 mL/min) [see Warnings and Precautions (5.3) and Use in Specific Populations (8.6)].
- Active major bleeding.
- Bacterial endocarditis.
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of fondaparinux sodium.
- Body weight <50 kg (venous thromboembolism [VTE] prophylaxis only) [see Warnings and Precautions (5.4)].
- History of serious hypersensitivity reaction (e.g., angioedema, anaphylactoid/anaphylactic reactions) to ARIXTRA.
-
5 WARNINGS AND PRECAUTIONS
5.1 Neuraxial Anesthesia and Post-operative Indwelling Epidural Catheter Use
Spinal or epidural hematomas, which may result in long-term or permanent paralysis, can occur with the use of anticoagulants and neuraxial (spinal/epidural) anesthesia or spinal puncture. The risk of these events may be higher with post-operative use of indwelling epidural catheters or concomitant use of other drugs affecting hemostasis such as NSAIDs [see Boxed Warning]. In the postmarketing experience, epidural or spinal hematoma has been reported in association with the use of ARIXTRA by subcutaneous (SC) injection. Optimal timing between the administration of ARIXTRA and neuraxial procedures is not known. Monitor patients undergoing these procedures for signs and symptoms of neurologic impairment such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), and bowel or bladder dysfunction. Consider the potential risks and benefits before neuraxial intervention in patients anticoagulated or who may be anticoagulated for thromboprophylaxis.
5.2 Hemorrhage
ARIXTRA increases the risk of hemorrhage in patients at risk for bleeding, including conditions such as congenital or acquired bleeding disorders, active ulcerative and angiodysplastic gastrointestinal disease, hemorrhagic stroke, uncontrolled arterial hypertension, diabetic retinopathy, or shortly after brain, spinal, or ophthalmological surgery. Cases of elevated aPTT temporally associated with bleeding events have been reported following administration of ARIXTRA (with or without concomitant administration of other anticoagulants) [see Adverse Reactions (6.5)].
Do not administer agents that enhance the risk of hemorrhage with ARIXTRA unless essential for the management of the underlying condition, such as vitamin K antagonists for the treatment of VTE. If co-administration is essential, closely monitor patients for signs and symptoms of bleeding.
Do not administer the initial dose of ARIXTRA earlier than 6 to 8 hours after surgery. Administration earlier than 6 hours after surgery increases risk of major bleeding [see Dosage and Administration (2) and Adverse Reactions (6.1)].
5.3 Renal Impairment and Bleeding Risk
ARIXTRA increases the risk of bleeding in patients with impaired renal function due to reduced clearance [see Clinical Pharmacology (12.4)].
The incidence of major bleeding by renal function status reported in clinical trials of patients receiving ARIXTRA for VTE surgical prophylaxis is provided in Table 1. In these patient populations, the following is recommended:
- Do not use ARIXTRA for VTE prophylaxis and treatment in patients with CrCl <30 mL/min [see Contraindications (4)].
- ARIXTRA may cause prolonged anticoagulation in patients with CrCl 30 to 50 mL/min.
Table 1. Incidence of Major Bleeding in Patients Treated With ARIXTRA by Renal Function Status for Surgical Prophylaxis and Treatment of Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE) CrCl = creatinine clearance. - * Hip fracture, hip replacement, and knee replacement surgery prophylaxis.
Degree of Renal Impairment
Population
Timing of Dose
Normal
%
(n/N)
Mild
%
(n/N)
Moderate
%
(n/N)
Severe
%
(n/N)
CrCl (mL/min)
≥80
≥50 - <80
≥30 - <50
<30
Orthopedic surgery*
Overall
1.6%
(25/1,565)
2.4%
(31/1,288)
3.8%
(19/504)
4.8%
(4/83)
6-8 hours after surgery
1.8%
(16/905)
2.2%
(15/675)
2.3%
(6/265)
0%
(0/40)
Abdominal surgery
Overall
2.1%
(13/606)
3.6%
(22/613)
6.7%
(12/179)
7.1%
(1/14)
6-8 hours after surgery
2.1%
(10/467)
3.3%
(16/481)
5.8%
(8/137)
7.7%
(1/13)
DVT and PE
Treatment
0.4%
(4/1,132)
1.6%
(12/733)
2.2%
(7/318)
7.3%
(4/55)
Assess renal function periodically in patients receiving ARIXTRA. Discontinue the drug immediately in patients who develop severe renal impairment while on therapy. After discontinuation of ARIXTRA, its anticoagulant effects may persist for 2 to 4 days in patients with normal renal function (i.e., at least 3 to 5 half-lives). The anticoagulant effects of ARIXTRA may persist even longer in patients with renal impairment [see Clinical Pharmacology (12.4)].
5.4 Body Weight <50 kg and Bleeding Risk
ARIXTRA increases the risk for bleeding in patients who weigh less than 50 kg, compared to patients with higher weights.
In patients who weigh less than 50 kg:
- Do not administer ARIXTRA as prophylactic therapy for patients undergoing hip fracture, hip replacement, or knee replacement surgery and abdominal surgery [see Contraindications (4)].
During the randomized clinical trials of VTE prophylaxis in the peri-operative period following hip fracture, hip replacement, or knee replacement surgery and abdominal surgery, major bleeding occurred at a higher rate among patients with a body weight <50 kg compared to those with a body weight >50 kg (5.4% versus 2.1% in patients undergoing hip fracture, hip replacement, or knee replacement surgery; 5.3% versus 3.3% in patients undergoing abdominal surgery).
5.5 Thrombocytopenia
Thrombocytopenia can occur with the administration of ARIXTRA. Thrombocytopenia of any degree should be monitored closely. Discontinue ARIXTRA if the platelet count falls below 100,000/mm3. Moderate thrombocytopenia (platelet counts between 100,000/mm3 and 50,000/mm3) occurred at a rate of 3.0% in patients given ARIXTRA 2.5 mg in the peri-operative hip fracture, hip replacement, or knee replacement surgery and abdominal surgery clinical trials. Severe thrombocytopenia (platelet counts less than 50,000/mm3) occurred at a rate of 0.2% in patients given ARIXTRA 2.5 mg in these clinical trials. During extended prophylaxis, no cases of moderate or severe thrombocytopenia were reported.
Moderate thrombocytopenia occurred at a rate of 0.5% in patients given the ARIXTRA treatment regimen in the DVT and PE treatment clinical trials. Severe thrombocytopenia occurred at a rate of 0.04% in patients given the ARIXTRA treatment regimen in the DVT and PE treatment clinical trials.
Occurrences of thrombocytopenia with thrombosis that manifested similar to heparin-induced thrombocytopenia have been reported with the use of ARIXTRA in postmarketing experience [see Adverse Reactions (6.5)].
5.6 Monitoring: Laboratory Tests
Routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT) are relatively insensitive measures of the activity of ARIXTRA and international standards of heparin or LMWH are not calibrators to measure anti-Factor Xa activity of ARIXTRA. If unexpected changes in coagulation parameters or major bleeding occur during therapy with ARIXTRA, discontinue ARIXTRA.In postmarketing experience, occurrences of aPTT elevations have been reported following administration of ARIXTRA [see Adverse Reactions (6.5)].
Periodic routine complete blood counts (including platelet count), serum creatinine level, and stool occult blood tests are recommended during the course of treatment with ARIXTRA.
The anti-Factor Xa activity of fondaparinux sodium can be measured by anti-Xa assay using the appropriate calibrator (fondaparinux). The activity of fondaparinux sodium is expressed in milligrams (mg) of the fondaparinux and cannot be compared with activities of heparin or low molecular weight heparins [see Clinical Pharmacology (12.2, 12.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Spinal or epidural hematomas [see Warnings and Precautions (5.1)]
- Hemorrhage [see Warnings and Precautions (5.2)]
- Renal impairment and bleeding risk [see Warnings and Precautions (5.3)]
- Body weight <50 kg and bleeding risk [see Warnings and Precautions (5.4)]
- Thrombocytopenia [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reaction information below is based on data from 8,877 patients exposed to ARIXTRA in controlled trials of hip fracture, hip replacement, major knee, or abdominal surgeries, and DVT and PE treatment.
Hemorrhage
During administration of ARIXTRA, the most common adverse reactions were bleeding complications [see Warnings and Precautions (5.2)].
Hip Fracture, Hip Replacement, and Knee Replacement Surgery
The rates of major bleeding events reported during 3 active-controlled peri-operative VTE prophylaxis trials with enoxaparin sodium in hip fracture, hip replacement, or knee replacement surgery (N = 3,616) and in an extended VTE prophylaxis trial (n = 327) with ARIXTRA 2.5 mg are provided in Table 2.
Table 2. Bleeding Across Randomized, Controlled Hip Fracture, Hip Replacement, and Knee Replacement Surgery Studies - * Enoxaparin sodium dosing regimen: 30 mg every 12 hours or 40 mg once daily.
- † Not approved for use in patients undergoing hip fracture surgery.
- ‡ Major bleeding was defined as clinically overt bleeding that was (1) fatal, (2) bleeding at critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), (3) associated with re-operation at operative site, or (4) with a bleeding index (BI) ≥2.
- § BI ≥2: Overt bleeding associated only with a bleeding index (BI) ≥2 calculated as [number of whole blood or packed red blood cell units transfused + [(pre-bleeding) – (post-bleeding)] hemoglobin (g/dL) values].
- ¶ Minor bleeding was defined as clinically overt bleeding that was not major.
Peri-Operative Prophylaxis
(Day 1 to Day 7 ± 1 post-surgery)Extended Prophylaxis
(Day 8 to Day 28 ± 2 post-surgery)ARIXTRA
2.5 mg SC
once daily
N = 3,616
N = 3,956
ARIXTRA
2.5 mg SC
once daily
N = 327
Placebo
SC once daily
N = 329
Major bleeding‡
96 (2.7%)
75 (1.9%)
8 (2.4%)
2 (0.6%)
Hip fracture
18/831 (2.2%)
19/842 (2.3%)
8/327 (2.4%)
2/329 (0.6%)
Hip replacement
67/2,268 (3.0%)
55/2,597 (2.1%)
—
—
Knee replacement
11/517 (2.1%)
1/517 (0.2%)
—
—
Fatal bleeding
0 (0.0%)
1 (<0.1%)
0 (0.0%)
0 (0.0%)
Non-fatal bleeding at critical site
0 (0.0%)
1 (<0.1%)
0 (0.0%)
0 (0.0%)
Re-operation due to bleeding
12 (0.3%)
10 (0.3%)
2 (0.6%)
2 (0.6%)
BI ≥2§
84 (2.3%)
63 (1.6%)
6 (1.8%)
0 (0.0%)
Minor bleeding¶
109 (3.0%)
116 (2.9%)
5 (1.5%)
2 (0.6%)
A separate analysis of major bleeding across all randomized, controlled, peri-operative, prophylaxis clinical studies of hip fracture, hip replacement, or knee replacement surgery according to the time of the first injection of ARIXTRA after surgical closure was performed in patients who received ARIXTRA only post-operatively. In this analysis, the incidences of major bleeding were as follows: <4 hours was 4.8% (5/104), 4 to 6 hours was 2.3% (28/1,196), 6 to 8 hours was 1.9% (38/1,965). In all studies, the majority (≥75%) of the major bleeding events occurred during the first 4 days after surgery.
Abdominal Surgery
In a randomized study of patients undergoing abdominal surgery, ARIXTRA 2.5 mg once daily (n = 1,433) was compared with dalteparin 5,000 IU once daily (n = 1,425). Bleeding rates are shown in Table 3.
Table 3. Bleeding in the Abdominal Surgery Study - * Major bleeding was defined as bleeding that was (1) fatal, (2) bleeding at the surgical site leading to intervention, (3) non-surgical bleeding at a critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), or leading to an intervention, and/or with a bleeding index (BI) ≥2.
- † Minor bleeding was defined as clinically overt bleeding that was not major.
ARIXTRA
2.5 mg SC once daily
Dalteparin Sodium
5,000 IU SC once daily
N = 1,433
N = 1,425
Major bleeding*
49 (3.4%)
34 (2.4%)
Fatal bleeding
2 (0.1%)
2 (0.1%)
Non-fatal bleeding at critical site
0 (0.0%)
0 (0.0%)
Other non-fatal major bleeding
Surgical site
38 (2.7%)
26 (1.8%)
Non-surgical site
9 (0.6%)
6 (0.4%)
Minor bleeding†
31 (2.2%)
23 (1.6%)
The rates of major bleeding according to the time interval following the first ARIXTRA injection were as follows: <6 hours was 3.4% (9/263) and 6 to 8 hours was 2.9% (32/1112).
Treatment of Deep Vein Thrombosis and Pulmonary Embolism
The rates of bleeding events reported during a dose-response trial (n = 111) and an active-controlled trial with enoxaparin sodium in DVT treatment (n = 1,091) and an active-controlled trial with heparin in PE treatment (n = 1,092) with ARIXTRA are provided in Table 4.
Table 4. Bleeding* in Deep Vein Thrombosis and Pulmonary Embolism Treatment Studies - * Bleeding rates are during the study drug treatment period (approximately 7 days). Patients were also treated with vitamin K antagonists initiated within 72 hours after the first study drug administration.
- † Major bleeding was defined as clinically overt: –and/or contributing to death – and/or in a critical organ including intracranial, retroperitoneal, intraocular, spinal, pericardial, or adrenal gland – and/or associated with a fall in hemoglobin level ≥2 g/dL – and/or leading to a transfusion ≥2 units of packed red blood cells or whole blood.
- ‡ Clinically overt bleeding with a 2 g/dL fall in hemoglobin and/or leading to transfusion of PRBC or whole blood ≥2 units.
- § Minor bleeding was defined as clinically overt bleeding that was not major.
ARIXTRA
N = 2,294
Enoxaparin Sodium
N = 1,101
Heparin
aPTT adjusted IV
N = 1,092
Major bleeding†
28 (1.2%)
13 (1.2%)
12 (1.1%)
Fatal bleeding
3 (0.1%)
0 (0.0%)
1 (0.1%)
Non-fatal bleeding at a critical site
3 (0.1%)
0 (0.0%)
2 (0.2%)
Intracranial bleeding
3 (0.1%)
0 (0.0%)
1 (0.1%)
Retro-peritoneal bleeding
0 (0.0%)
0 (0.0%)
1 (0.1%)
Other clinically overt bleeding‡
22 (1.0%)
13 (1.2%)
10 (0.9%)
Minor bleeding§
70 (3.1%)
33 (3.0%)
57 (5.2%)
6.2 Local Reactions
Local irritation (injection site bleeding, rash, and pruritus) may occur following subcutaneous injection of ARIXTRA.
6.3 Elevations of Serum Aminotransferases
In the peri-operative prophylaxis randomized clinical trials of 7 ± 2 days, asymptomatic increases in aspartate (AST) and alanine (ALT) aminotransferase levels greater than 3 times the upper limit of normal were reported in 1.7% and 2.6% of patients, respectively, during treatment with ARIXTRA 2.5 mg once daily versus 3.2% and 3.9% of patients, respectively, during treatment with enoxaparin sodium 30 mg every 12 hours or 40 mg once daily enoxaparin sodium. These elevations are reversible and may be associated with increases in bilirubin. In the extended prophylaxis clinical trial, no significant differences in AST and ALT levels between ARIXTRA 2.5 mg and placebo-treated patients were observed.
In the DVT and PE treatment clinical trials, asymptomatic increases in AST and ALT levels greater than 3 times the upper limit of normal of the laboratory reference range were reported in 0.7% and 1.3% of patients, respectively, during treatment with ARIXTRA. In comparison, these increases were reported in 4.8% and 12.3% of patients, respectively, in the DVT treatment trial during treatment with enoxaparin sodium 1 mg/kg every 12 hours and in 2.9% and 8.7% of patients, respectively, in the PE treatment trial during treatment with aPTT adjusted heparin.
Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, elevations that might be caused by drugs like ARIXTRA should be interpreted with caution.
6.4 Other Adverse Reactions
Other adverse reactions that occurred during treatment with ARIXTRA in clinical trials with patients undergoing hip fracture, hip replacement, or knee replacement surgery are provided in Table 5.
Table 5. Adverse Reactions Across Randomized, Controlled, Hip Fracture Surgery, Hip Replacement Surgery, and Knee Replacement Surgery Studies - * Enoxaparin sodium dosing regimen: 30 mg every 12 hours or 40 mg once daily.
- † Not approved for use in patients undergoing hip fracture surgery.
- ‡ Localized blister coded as bullous eruption.
Adverse Reactions
Peri-Operative Prophylaxis
(Day 1 to Day 7 ± 1 post-surgery)Extended Prophylaxis
(Day 8 to Day 28 ± 2 post-surgery)ARIXTRA
2.5 mg SC
once daily
ARIXTRA
2.5 mg SC
once daily
Placebo
SC once daily
N = 3,616
N = 3,956
N = 327
N = 329
Anemia
707 (19.6%)
670 (16.9%)
5 (1.5%)
4 (1.2%)
Insomnia
179 (5.0%)
214 (5.4%)
3 (0.9%)
1 (0.3%)
Wound drainage increased
161 (4.5%)
184 (4.7%)
2 (0.6%)
0 (0.0%)
Hypokalemia
152 (4.2%)
164 (4.1%)
0 (0.0%)
0 (0.0%)
Dizziness
131 (3.6%)
165 (4.2%)
2 (0.6%)
0 (0.0%)
Purpura
128 (3.5%)
137 (3.5%)
0 (0.0%)
0 (0.0%)
Hypotension
126 (3.5%)
125 (3.2%)
1 (0.3%)
0 (0.0%)
Confusion
113 (3.1%)
132 (3.3%)
4 (1.2%)
1 (0.3%)
Bullous eruption‡
112 (3.1%)
102 (2.6%)
0 (0.0%)
1 (0.3%)
Hematoma
103 (2.8%)
109 (2.8%)
7 (2.1%)
1 (0.3%)
Post-operative hemorrhage
85 (2.4%)
69 (1.7%)
2 (0.6%)
2 (0.6%)
The most common adverse reaction in the abdominal surgery trial was post-operative wound infection (4.9%), and the most common adverse reaction in the VTE treatment trials was epistaxis (1.3%).
6.5 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ARIXTRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In the postmarketing experience, epidural or spinal hematoma has been reported in association with the use of ARIXTRA by subcutaneous (SC) injection [see Warnings and Precautions (5.1)]. Occurrences of thrombocytopenia with thrombosis that manifested similar to heparin-induced thrombocytopenia have been reported in the postmarketing experience and cases of elevated aPTT temporally associated with bleeding events have been reported following administration of ARIXTRA (with or without concomitant administration of other anticoagulants) [see Warnings and Precautions (5.5)].
Serious allergic reactions, including angioedema, anaphylactoid/anaphylactic reactions have been reported with the use of ARIXTRA [see Contraindications (4)].
-
7 DRUG INTERACTIONS
In clinical studies performed with ARIXTRA, the concomitant use of oral anticoagulants (warfarin), platelet inhibitors (acetylsalicylic acid), NSAIDs (piroxicam), and digoxin did not significantly affect the pharmacokinetics/pharmacodynamics of fondaparinux sodium. In addition, ARIXTRA neither influenced the pharmacodynamics of warfarin, acetylsalicylic acid, piroxicam, and digoxin, nor the pharmacokinetics of digoxin at steady state.
Agents that may enhance the risk of hemorrhage should be discontinued prior to initiation of therapy with ARIXTRA unless these agents are essential. If co-administration is necessary, monitor patients closely for hemorrhage [see Warnings and Precautions (5.2)].
In an in vitro study in human liver microsomes, inhibition of CYP2A6 hydroxylation of coumarin by fondaparinux (200 micromolar i.e., 350 mg/L) was 17 to 28%. Inhibition of the other isozymes evaluated (CYPs 1A2, 2C9, 2C19, 2D6, 3A4, and 3E1) was 0 to 16%. Since fondaparinux does not markedly inhibit CYP450s (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4) in vitro,fondaparinux sodium is not expected to significantly interact with other drugs in vivo by inhibition of metabolism mediated by these isozymes.
Since fondaparinux sodium does not bind significantly to plasma proteins other than ATIII, no drug interactions by protein-binding displacement are expected.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published literature and postmarketing reports have not reported a clear association with fondaparinux sodium and adverse developmental outcomes. Fondaparinux sodium plasma concentrations obtained from four women treated with ARIXTRA during pregnancy and their newborn infants demonstrated low placental transfer of fondaparinux sodium (see Data). There are risks to the mother associated with untreated venous thromboembolism in pregnancy and a risk of hemorrhage in the mother and fetus associated with use of anticoagulants (see Clinical Considerations). In animal reproduction studies, there was no evidence of adverse developmental outcomes when fondaparinux sodium was administered to pregnant rats and rabbits during organogenesis at doses 32 and 65 times, respectively, the recommended human dose based on body surface area.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Disease-associated maternal and/or embryo/fetal risk
Pregnancy confers an increased risk for thromboembolism that is higher for women with underlying thromboembolic disease and certain high-risk pregnancy conditions. Published data describe that women with a previous history of venous thrombosis are at high risk for recurrence during pregnancy.
Fetal/Neonatal adverse reactions
Fondaparinux sodium has been demonstrated to cross the placenta in humans (see Data). Use of anticoagulants, including fondaparinux sodium, may increase the risk of bleeding in the fetus and neonate. Monitor neonates for bleeding [see Warnings and Precautions (5.2, 5.4, 5.6)].
Labor or delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding. Fondaparinux sodium use during labor or delivery in women who are receiving neuraxial anesthesia may result in epidural or spinal hematomas. Pregnant women receiving fondaparinux sodium should be carefully monitored for evidence of bleeding or unexpected changes in coagulation parameters. Consideration for use of a shorter acting anticoagulant should be specifically addressed as delivery approaches [see Warnings and Precautions (5.1, 5.6)].
Human Data
In a study of five pregnant women treated with fondaparinux sodium during the third trimester of pregnancy at a dose of 2.5 mg/day, four of the women had elevated anti-factor Xa activity noted in the cord blood. Anti-factor Xa clotting times in these four cases were between 37.5 and 50.9 seconds. The patient who did not have elevated anti-factor Xa activity had received only one dose of fondaparinux sodium 22 hours prior to delivery. The concentration of fondaparinux sodium in umbilical cord plasma was approximately 1/10th the level of fondaparinux sodium in maternal plasma. None of the infants experienced adverse effects.
Animal Data
Embryo-fetal development studies have been conducted with fondaparinux sodium in pregnant rats at subcutaneous doses up to 10 mg/kg/day (about 32 times the recommended human dose based on body surface area) administered from days 6 to 17 of gestation and pregnant rabbits at subcutaneous doses up to 10 mg/kg/day (about 65 times the recommended human dose based on body surface area) administered from days 6 to 18 of gestation. These studies have revealed no evidence of adverse developmental outcomes when fondaparinux sodium was administered to pregnant rats and rabbits during organogenesis. Additionally, there were no effects on pre and postnatal development in a study conducted in rats at subcutaneous doses up to 10 mg/kg/day (about 32 times the recommended human dose based on body surface area).
8.2 Lactation
Risk Summary
There are no data on the presence of fondaparinux sodium in human milk, or the effects on milk production. Limited clinical data during lactation preclude a clear determination of the risk of ARIXTRA to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ARIXTRA and any potential adverse effects on the breastfed infant from ARIXTRA or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of ARIXTRA in pediatric patients have not been established. Because risk for bleeding during treatment with ARIXTRA is increased in adults who weigh <50 kg, bleeding may be a particular safety concern for use of ARIXTRA in the pediatric population [see Warnings and Precautions (5.4)].
8.5 Geriatric Use
In clinical trials the efficacy of ARIXTRA in the elderly (65 years or older) was similar to that seen in patients younger than 65 years; however, serious adverse events increased with age. When using ARIXTRA in elderly patients, paying particular attention to dosing directions and concomitant medications (especially anti-platelet medication) [see Warnings and Precautions (5.2)].
Fondaparinux sodium is substantially excreted by the kidney, and the risk of adverse reactions to ARIXTRA may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, assess renal function prior to ARIXTRA administration [see Contraindications (4), Warnings and Precautions (5.3), and Clinical Pharmacology (12.4)].
In the peri-operative hip fracture, hip replacement, or knee replacement surgery clinical trials with patients receiving ARIXTRA 2.5 mg, serious adverse events increased with age for patients receiving ARIXTRA. The incidence of major bleeding in clinical trials of ARIXTRA by age is provided in Table 6.
Table 6. Incidence of Major Bleeding in Patients Treated With ARIXTRA by Age - * Includes hip fracture, hip replacement, and knee replacement surgery prophylaxis.
Age
<65 years
% (n/N)
65 to 74 years
% (n/N)
≥75 years
% (n/N)
Orthopedic surgery*
1.8% (23/1,253)
2.2% (24/1,111)
2.7% (33/1,277)
Extended prophylaxis
1.9% (1/52)
1.4% (1/71)
2.9% (6/204)
Abdominal surgery
3.0% (19/644)
3.2% (16/507)
5.0% (14/282)
DVT and PE treatment
0.6% (7/1,151)
1.6% (9/560)
2.1% (12/583)
8.6 Renal Impairment
Patients with impaired renal function are at increased risk of bleeding due to reduced clearance of ARIXTRA [see Contraindications (4) and Warnings and Precautions (5.3)]. Assess renal function periodically in patients receiving ARIXTRA. Discontinue ARIXTRA immediately in patients who develop severe renal impairment while on therapy. After discontinuation of ARIXTRA, its anticoagulant effects may persist for 2 to 4 days in patients with normal renal function (i.e., at least 3 to 5 half-lives). The anticoagulant effects of ARIXTRA may persist even longer in patients with renal impairment [see Clinical Pharmacology (12.4)].
8.7 Hepatic Impairment
Following a single, subcutaneous dose of 7.5 mg of ARIXTRA in patients with moderate hepatic impairment (Child-Pugh Category B) compared to subjects with normal liver function, changes from baseline in aPTT, PT/INR, and antithrombin III were similar in the two groups. However, a higher incidence of hemorrhage was observed in subjects with moderate hepatic impairment than in normal subjects, especially mild hematomas at the blood sampling or injection site. The pharmacokinetics of fondaparinux have not been studied in patients with severe hepatic impairment [see Dosage and Administration (2.4) and Clinical Pharmacology (12.4)].
-
10 OVERDOSAGE
There is no known antidote for ARIXTRA. Overdose of ARIXTRA may lead to hemorrhagic complications. Discontinue treatment and initiate appropriate therapy if bleeding complications associated with overdosage occur.
Data obtained in patients undergoing chronic intermittent hemodialysis suggest that clearance of ARIXTRA can increase by 20% during hemodialysis.
-
11 DESCRIPTION
ARIXTRA (fondaparinux sodium injection, USP) is a sterile solution containing fondaparinux sodium. It is a synthetic and specific inhibitor of activated Factor X (Xa). Fondaparinux sodium is methyl O-2-deoxy-6-O-sulfo-2-(sulfoamino)-α-D-glucopyranosyl-(1→4)-O-β-D-glucopyra-nuronosyl-(1→4)-O-2-deoxy-3,6-di-O-sulfo-2-(sulfoamino)-α-D-glucopyranosyl-(1→4)-O-2-O-sulfo-α-L-idopyranuronosyl-(1→4)-2-deoxy-6-O-sulfo-2-(sulfoamino)-α-D-glucopyranoside, decasodium salt.
The molecular formula of fondaparinux sodium is C31H43N3Na10O49S8 and its molecular weight is 1728. The structural formula is provided below:
ARIXTRA is supplied as a sterile, preservative-free injectable solution for subcutaneous use.
Each single-dose, prefilled syringe of ARIXTRA, affixed with an automatic needle protection system, contains 2.5 mg of fondaparinux sodium in 0.5 mL, 5.0 mg of fondaparinux sodium in 0.4 mL, 7.5 mg of fondaparinux sodium in 0.6 mL, or 10.0 mg of fondaparinux sodium in 0.8 mL of an isotonic solution of sodium chloride and water for injection. The final drug product is a clear and colorless to slightly yellow liquid with a pH between 5.0 and 8.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The antithrombotic activity of fondaparinux sodium is the result of antithrombin III (ATIII)-mediated selective inhibition of Factor Xa. By selectively binding to ATIII, fondaparinux sodium potentiates (about 300 times) the innate neutralization of Factor Xa by ATIII. Neutralization of Factor Xa interrupts the blood coagulation cascade and thus inhibits thrombin formation and thrombus development.
Fondaparinux sodium does not inactivate thrombin (activated Factor II) and has no known effect on platelet function. At the recommended dose, fondaparinux sodium does not affect fibrinolytic activity or bleeding time.
12.2 Pharmacodynamics
Anti-Xa Activity
The pharmacodynamics/pharmacokinetics of fondaparinux sodium are derived from fondaparinux plasma concentrations quantified via anti-Factor Xa activity. Only fondaparinux can be used to calibrate the anti-Xa assay. (The international standards of heparin or LMWH are not appropriate for this use.) As a result, the activity of fondaparinux sodium is expressed as milligrams (mg) of the fondaparinux calibrator. The anti-Xa activity of the drug increases with increasing drug concentration, reaching maximum values in approximately three hours.
12.3 Pharmacokinetics
Absorption
Fondaparinux sodium administered by subcutaneous injection is rapidly and completely absorbed (absolute bioavailability is 100%). Following a single subcutaneous dose of fondaparinux sodium 2.5 mg in young male subjects, Cmax of 0.34 mg/L is reached in approximately 2 hours. In patients undergoing treatment with fondaparinux sodium injection 2.5 mg, once daily, the peak steady-state plasma concentration is, on average, 0.39 to 0.50 mg/L and is reached approximately 3 hours post-dose. In these patients, the minimum steady-state plasma concentration is 0.14 to 0.19 mg/L. In patients with symptomatic deep vein thrombosis and pulmonary embolism undergoing treatment with fondaparinux sodium injection 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), and 10 mg (body weight >100 kg) once daily, the body–weight-adjusted doses provide similar mean steady-state peaks and minimum plasma concentrations across all body weight categories. The mean peak steady-state plasma concentration is in the range of 1.20 to 1.26 mg/L. In these patients, the mean minimum steady-state plasma concentration is in the range of 0.46 to 0.62 mg/L.
Distribution
In healthy adults, intravenously or subcutaneously administered fondaparinux sodium distributes mainly in blood and only to a minor extent in extravascular fluid as evidenced by steady state and non-steady state apparent volume of distribution of 7 to 11 L. Similar fondaparinux distribution occurs in patients undergoing elective hip surgery or hip fracture surgery. In vitro, fondaparinux sodium is highly (at least 94%) and specifically bound to antithrombin III (ATIII) and does not bind significantly to other plasma proteins (including platelet Factor 4 [PF4]) or red blood cells.
Metabolism
In vivo metabolism of fondaparinux has not been investigated since the majority of the administered dose is eliminated unchanged in urine in individuals with normal kidney function.
Elimination
In individuals with normal kidney function, fondaparinux is eliminated in urine mainly as unchanged drug. In healthy individuals up to 75 years of age, up to 77% of a single subcutaneous or intravenous fondaparinux dose is eliminated in urine as unchanged drug in 72 hours. The elimination half-life is 17 to 21 hours.
12.4 Special Populations
Renal Impairment
Fondaparinux elimination is prolonged in patients with renal impairment since the major route of elimination is urinary excretion of unchanged drug. In patients undergoing prophylaxis following elective hip surgery or hip fracture surgery, the total clearance of fondaparinux is approximately 25% lower in patients with mild renal impairment (CrCl 50 to 80 mL/min), approximately 40% lower in patients with moderate renal impairment (CrCl 30 to 50 mL/min), and approximately 55% lower in patients with severe renal impairment (<30 mL/min) compared to patients with normal renal function. A similar relationship between fondaparinux clearance and extent of renal impairment was observed in DVT treatment patients [see Contraindications (4) and Warnings and Precautions (5.3)].
Hepatic Impairment
Following a single, subcutaneous dose of 7.5 mg of ARIXTRA in patients with moderate hepatic impairment (Child-Pugh Category B), Cmax and AUC were decreased by 22% and 39%, respectively, compared to subjects with normal liver function. The changes from baseline in pharmacodynamic parameters, such as aPTT, PT/INR, and antithrombin III, were similar in normal subjects and in patients with moderate hepatic impairment. Based on these data, no dosage adjustment is recommended in these patients. However, a higher incidence of hemorrhage was observed in subjects with moderate hepatic impairment than in normal subjects [see Use in Specific Populations (8.7)]. The pharmacokinetics of fondaparinux have not been studied in patients with severe hepatic impairment [see Dosage and Administration (2.4)].
Pediatric
The pharmacokinetics of fondaparinux have not been investigated in pediatric patients [see Contraindications (4), Warnings and Precautions (5.4), and Pediatric Use (8.4)].
Geriatric
Fondaparinux elimination is prolonged in patients older than 75 years. In studies evaluating fondaparinux sodium 2.5 mg prophylaxis in hip fracture surgery or elective hip surgery, the total clearance of fondaparinux was approximately 25% lower in patients older than 75 years as compared to patients younger than 65 years. A similar relationship between fondaparinux clearance and age was observed in DVT treatment patients [see Use in Specific Populations (8.5)].
Patients Weighing Less Than 50 kg
Total clearance of fondaparinux sodium is decreased by approximately 30% in patients weighing less than 50 kg [see Dosage and Administration (2.3) and Contraindications (4)].
Gender
The pharmacokinetic properties of fondaparinux sodium are not significantly affected by gender.
Race
Pharmacokinetic differences due to race have not been studied prospectively. However, studies performed in Asian (Japanese) healthy subjects did not reveal a different pharmacokinetic profile compared to Caucasian healthy subjects. Similarly, no plasma clearance differences were observed between black and Caucasian patients undergoing orthopedic surgery.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of fondaparinux sodium.
Fondaparinux sodium was not genotoxic in the Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, the human lymphocyte chromosome aberration test, the rat hepatocyte unscheduled DNA synthesis (UDS) test, or the rat micronucleus test.
At subcutaneous doses up to 10 mg/kg/day (about 32 times the recommended human dose based on body surface area), fondaparinux sodium was found to have no effect on fertility and reproductive performance of male and female rats.
-
14 CLINICAL STUDIES
14.1 Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery
In a randomized, double-blind, clinical trial in patients undergoing hip fracture surgery, ARIXTRA 2.5 mg SC once daily was compared to enoxaparin sodium 40 mg SC once daily, which is not approved for use in patients undergoing hip fracture surgery. A total of 1,711 patients were randomized and 1,673 were treated. Patients ranged in age from 17 to 101 years (mean age 77 years) with 25% men and 75% women. Patients were 99% Caucasian, 1% other races. Patients with multiple traumas affecting more than one organ system, serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. ARIXTRA was initiated after surgery in 88% of patients (mean 6 hours) and enoxaparin sodium was initiated after surgery in 74% of patients (mean 18 hours). For both drugs, treatment was continued for 7 ± 2 days. The primary efficacy endpoint, venous thromboembolism (VTE), was a composite of documented deep vein thrombosis (DVT) and/or documented symptomatic pulmonary embolism (PE) reported up to Day 11. The efficacy data are provided in Table 7 and demonstrate that under the conditions of the trial ARIXTRA was associated with a VTE rate of 8.3% compared with a VTE rate of 19.1% for enoxaparin sodium for a relative risk reduction of 56% (95% CI: 39%, 70%; P <0.001). Major bleeding episodes occurred in 2.2% of patients receiving ARIXTRA and 2.3% of enoxaparin sodium patients [see Adverse Reactions (6.1)].
Table 7. Efficacy of ARIXTRA in the Peri-operative Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery - * N = all evaluable hip fracture surgery patients. Evaluable patients were those who were treated and underwent the appropriate surgery (i.e., hip fracture surgery of the upper third of the femur), with an adequate efficacy assessment up to Day 11.
- † P value versus enoxaparin sodium <0.001.
- ‡ P value versus enoxaparin sodium: NS.
Endpoint
Peri-operative Prophylaxis
(Day 1 to Day 7 ± 2 post-surgery)
ARIXTRA
2.5 mg SC once daily
Enoxaparin Sodium
40 mg SC once daily
n/N*
% (95% CI)
n/N*
% (95% CI)
VTE
52/626
8.3%† (6.3, 10.8)
119/624
19.1% (16.1, 22.4)
All DVT
49/624
7.9%† (5.9, 10.2)
117/623
18.8% (15.8, 22.1)
Proximal DVT
6/650
0.9%† (0.3, 2.0)
28/646
4.3% (2.9, 6.2)
Symptomatic PE
3/831
0.4%‡ (0.1, 1.1)
3/840
0.4% (0.1, 1.0)
14.2 Extended Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery
In a noncomparative, unblinded manner, 737 patients undergoing hip fracture surgery were initially treated during the peri-operative period with ARIXTRA 2.5 mg once daily for 7 ± 1 days. Eighty-one (81) of the 737 patients were not eligible for randomization into the 3‑week double-blind period. Three hundred twenty-six (326) patients and 330 patients were randomized to receive ARIXTRA 2.5 mg once daily or placebo, respectively, in or out of the hospital for 21 ± 2 days. Patients ranged in age from 23 to 96 years (mean age 75 years) and were 29% men and 71% women. Patients were 99% Caucasian and 1% other races. Patients with multiple traumas affecting more than one organ system or serum creatinine level more than 2 mg/dL (180 micromol/L) were excluded from the trial. The primary efficacy endpoint, venous thromboembolism (VTE), was a composite of documented deep vein thrombosis (DVT) and/or documented symptomatic pulmonary embolism (PE) reported for up to 24 days following randomization. The efficacy data are provided in Table 8 and demonstrate that extended prophylaxis with ARIXTRA was associated with a VTE rate of 1.4% compared with a VTE rate of 35.0% for placebo for a relative risk reduction of 95.9% (95% CI = [98.7; 87.1], P <0.0001). Major bleeding rates during the 3-week extended prophylaxis period for ARIXTRA occurred in 2.4% of patients receiving ARIXTRA and 0.6% of placebo-treated patients [see Adverse Reactions (6.1)].
Table 8. Efficacy of ARIXTRA Injection in the Extended Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery - * N = all randomized evaluable hip fracture surgery patients. Evaluable patients were those who were treated in the post-randomization period, with an adequate efficacy assessment for up to 24 days following randomization.
- † P value versus placebo <0.001
- ‡ P value versus placebo = 0.021.
- § P value versus placebo = NS.
Endpoint
Extended Prophylaxis
(Day 8 to Day 28 ± 2 post-surgery)
ARIXTRA
2.5 mg SC once daily
Placebo
SC once daily
n/N*
% (95% CI)
n/N*
% (95% CI)
VTE
3/208
1.4%† (0.3, 4.2)
77/220
35.0% (28.7, 41.7)
All DVT
3/208
1.4%†(0.3, 4.2)
74/218
33.9% (27.7, 40.6)
Proximal DVT
2/221
0.9%†(0.1, 3.2)
35/222
15.8% (11.2, 21.2)
Symptomatic VTE (all)
1/326
0.3%‡ (0.0, 1.7)
9/330
2.7% (1.3, 5.1)
Symptomatic PE
0/326
0.0%§(0.0, 1.1)
3/330
0.9% (0.2, 2.6)
14.3 Prophylaxis of Thromboembolic Events Following Hip Replacement Surgery
In 2 randomized, double-blind, clinical trials in patients undergoing hip replacement surgery, ARIXTRA 2.5 mg SC once daily was compared to either enoxaparin sodium 30 mg SC every 12 hours (Study 1) or to enoxaparin sodium 40 mg SC once a day (Study 2). In Study 1, a total of 2,275 patients were randomized and 2,257 were treated. Patients ranged in age from 18 to 92 years (mean age 65 years) with 48% men and 52% women. Patients were 94% Caucasian, 4% black, <1% Asian, and 2% others. In Study 2, a total of 2,309 patients were randomized and 2,273 were treated. Patients ranged in age from 24 to 97 years (mean age 65 years) with 42% men and 58% women. Patients were 99% Caucasian, and 1% other races. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from both trials. In Study 1, ARIXTRA was initiated 6 ± 2 hours (mean 6.5 hours) after surgery in 92% of patients and enoxaparin sodium was initiated 12 to 24 hours (mean 20.25 hours) after surgery in 97% of patients. In Study 2, ARIXTRA was initiated 6 ± 2 hours (mean 6.25 hours) after surgery in 86% of patients and enoxaparin sodium was initiated 12 hours before surgery in 78% of patients. The first post-operative enoxaparin sodium dose was given within 12 hours after surgery in 60% of patients and 12 to 24 hours after surgery in 35% of patients with a mean of 13 hours. For both studies, both study treatments were continued for 7 ± 2 days. The efficacy data are provided in Table 9. Under the conditions of Study 1, ARIXTRA was associated with a VTE rate of 6.1% compared with a VTE rate of 8.3% for enoxaparin sodium for a relative risk reduction of 26% (95% CI: -11%, 53%; P = NS). Under the conditions of Study 2, fondaparinux sodium was associated with a VTE rate of 4.1% compared with a VTE rate of 9.2% for enoxaparin sodium for a relative risk reduction of 56% (95% CI: 33%, 73%; P <0.001). For the 2 studies combined, the major bleeding episodes occurred in 3.0% of patients receiving ARIXTRA and 2.1% of enoxaparin sodium patients [see Adverse Reactions (6.1)].
Table 9. Efficacy of ARIXTRA in the Prophylaxis of Thromboembolic Events Following Hip Replacement Surgery - * N = all evaluable hip replacement surgery patients. Evaluable patients were those who were treated and underwent the appropriate surgery (i.e., hip replacement surgery), with an adequate efficacy assessment up to Day 11.
- † VTE was a composite of documented DVT and/or documented symptomatic PE reported up to Day 11.
- ‡ P value versus enoxaparin sodium: NS.
- § P value versus enoxaparin sodium in study 2: <0.001.
- ¶ P value versus enoxaparin sodium in study 1: <0.05.
- # P value versus enoxaparin sodium in study 2: <0.01.
Endpoint
Study 1
n/N*
% (95% CI)
Study 2
n/N*
% (95% CI)
ARIXTRA
2.5 mg SC
once daily
Enoxaparin
Sodium
30 mg SC
every 12 hr
ARIXTRA
2.5 mg SC
once daily
Enoxaparin
Sodium
40 mg SC
once daily
VTE†
48/787
6.1%‡ (4.5, 8.0)
66/797
8.3% (6.5, 10.4)
37/908
4.1%§ (2.9, 5.6)
85/919
9.2% (7.5, 11.3)
All DVT
44/784
5.6%¶ (4.1, 7.5)
65/796
8.2% (6.4, 10.3)
36/908
4.0%§ (2.8, 5.4)
83/918
9.0% (7.3, 11.1)
Proximal DVT
14/816
1.7%‡(0.9, 2.9)
10/830
1.2% (0.6, 2.2)
6/922
0.7%# (0.2, 1.4)
23/927
2.5% (1.6, 3.7)
Symptomatic PE
5/1,126
0.4%‡(0.1, 1.0)
1/1,128
0.1% (0.0, 0.5)
2/1,129
0.2%‡(0.0, 0.6)
2/1,123
0.2% (0.0, 0.6)
14.4 Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery
In a randomized, double-blind, clinical trial in patients undergoing knee replacement surgery (i.e., surgery requiring resection of the distal end of the femur or proximal end of the tibia), ARIXTRA 2.5 mg SC once daily was compared to enoxaparin sodium 30 mg SC every 12 hours. A total of 1,049 patients were randomized and 1,034 were treated. Patients ranged in age from 19 to 94 years (mean age 68 years) with 41% men and 59% women. Patients were 88% Caucasian, 8% black, <1% Asian, and 3% others. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. ARIXTRA was initiated 6 ± 2 hours (mean 6.25 hours) after surgery in 94% of patients, and enoxaparin sodium was initiated 12 to 24 hours (mean 21 hours) after surgery in 96% of patients. For both drugs, treatment was continued for 7 ± 2 days. The efficacy data are provided in Table 10 and demonstrate that under the conditions of the trial, ARIXTRA was associated with a VTE rate of 12.5% compared with a VTE rate of 27.8% for enoxaparin sodium for a relative risk reduction of 55% (95% CI: 36%, 70%; P <0.001). Major bleeding episodes occurred in 2.1% of patients receiving ARIXTRA and 0.2% of enoxaparin sodium patients [see Adverse Reactions (6.1)].
Table 10. Efficacy of ARIXTRA in the Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery - * N = all evaluable knee replacement surgery patients. Evaluable patients were those who were treated and underwent the appropriate surgery (i.e., knee replacement surgery), with an adequate efficacy assessment up to Day 11.
- † VTE was a composite of documented DVT and/or documented symptomatic PE reported up to Day 11.
- ‡ P value versus enoxaparin sodium <0.001.
- § P value versus enoxaparin sodium: NS.
Endpoint
ARIXTRA
2.5 mg SC once dailyEnoxaparin Sodium
30 mg SC every 12 hours
n/N*
% (95% CI)
n/N*
% (95% CI)
VTE†
45/361
12.5%‡ (9.2, 16.3)
101/363
27.8% (23.3, 32.7)
All DVT
45/361
12.5%‡ (9.2, 16.3)
98/361
27.1% (22.6, 32.0)
Proximal DVT
9/368
2.4%§ (1.1, 4.6)
20/372
5.4% (3.3, 8.2)
Symptomatic PE
1/517
0.2%§ (0.0, 1.1)
4/517
0.8% (0.2, 2.0)
14.5 Prophylaxis of Thromboembolic Events Following Abdominal Surgery in Patients at Risk for Thromboembolic Complications
Abdominal surgery patients at risk included the following: Those undergoing surgery under general anesthesia lasting longer than 45 minutes who are older than 60 years with or without additional risk factors; and those undergoing surgery under general anesthesia lasting longer than 45 minutes who are older than 40 years with additional risk factors. Risk factors included neoplastic disease, obesity, chronic obstructive pulmonary disease, inflammatory bowel disease, history of deep vein thrombosis (DVT) or pulmonary embolism (PE), or congestive heart failure.
In a randomized, double-blind, clinical trial in patients undergoing abdominal surgery, ARIXTRA 2.5 mg SC once daily started postoperatively was compared to dalteparin sodium 5,000 IU SC once daily, with one 2,500 IU SC preoperative injection and a 2,500 IU SC first postoperative injection. A total of 2,927 patients were randomized and 2,858 were treated. Patients ranged in age from 17 to 93 years (mean age 65 years) with 55% men and 45% women. Patients were 97% Caucasian, 1% black, 1% Asian, and 1% others. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. Sixty-nine percent (69%) of study patients underwent cancer-related abdominal surgery. Study treatment was continued for 7 ± 2 days. The efficacy data are provided in Table 11 and demonstrate that prophylaxis with ARIXTRA was associated with a VTE rate of 4.6% compared with a VTE rate of 6.1% for dalteparin sodium (P = NS).
Table 11. Efficacy of ARIXTRA In Prophylaxis of Thromboembolic Events Following Abdominal Surgery - * N = all evaluable abdominal surgery patients. Evaluable patients were those who were randomized and had an adequate efficacy assessment up to Day 10; non-treated patients and patients who did not undergo surgery did not get a VTE assessment.
- † VTE was a composite of venogram positive DVT, symptomatic DVT, non-fatal PE and/or fatal PE reported up to Day 10.
- ‡ P value versus dalteparin sodium: NS.
Endpoint
ARIXTRA
2.5 mg SC once daily
Dalteparin Sodium
5,000 IU SC once daily
n/N*
% (95% CI)
n/N*
% (95% CI)
VTE†
47/1,027
4.6%‡ (3.4, 6.0)
62/1,021
6.1% (4.7, 7.7)
All DVT
43/1,024
4.2% (3.1, 5.6)
59/1,018
5.8% (4.4, 7.4)
Proximal DVT
5/1,076
0.5% (0.2, 1.1)
5/1,077
0.5% (0.2, 1.1)
Symptomatic VTE
6/1,465
0.4% (0.2, 0.9)
5/1,462
0.3% (0.1, 0.8)
14.6 Treatment of Deep Vein Thrombosis
In a randomized, double-blind, clinical trial in patients with a confirmed diagnosis of acute symptomatic DVT without PE, ARIXTRA 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), or 10 mg (body weight >100 kg) SC once daily (ARIXTRA treatment regimen) was compared to enoxaparin sodium 1 mg/kg SC every 12 hours. Almost all patients started study treatment in hospital. Approximately 30% of patients in both groups were discharged home from the hospital while receiving study treatment. A total of 2,205 patients were randomized and 2,192 were treated. Patients ranged in age from 18 to 95 years (mean age 61 years) with 53% men and 47% women. Patients were 97% Caucasian, 2% black, and 1% other races. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. For both groups, treatment continued for at least 5 days with a treatment duration range of 7 ± 2 days, and both treatment groups received vitamin K antagonist therapy initiated within 72 hours after the first study drug administration and continued for 90 ± 7 days, with regular dose adjustments to achieve an INR of 2 to 3. The primary efficacy endpoint was confirmed, symptomatic, recurrent VTE reported up to Day 97. The efficacy data are provided in Table 12.
Table 12. Efficacy of ARIXTRA in the Treatment of Deep Vein Thrombosis (All Randomized) - * VTE was a composite of symptomatic recurrent non-fatal VTE or fatal PE reported up to Day 97. The 95% confidence interval for the treatment difference for total VTE was: (-1.8% to 1.5%).
Endpoint
ARIXTRA
5, 7.5, or 10 mg SC once daily
N = 1,098
Enoxaparin Sodium
1 mg/kg SC every 12 hours
N = 1,107
n
% (95% CI)
n
% (95% CI)
Total VTE*
43
3.9% (2.8, 5.2)
45
4.1% (3.0, 5.4)
DVT only
18
1.6% (1.0, 2.6)
28
2.5% (1.7, 3.6)
Non-fatal PE
20
1.8% (1.1, 2.8)
12
1.1% (0.6, 1.9)
Fatal PE
5
0.5% (0.1, 1.1)
5
0.5% (0.1, 1.1)
During the initial treatment period, 18 (1.6%) of patients treated with fondaparinux sodium and 10 (0.9%) of patients treated with enoxaparin sodium had a VTE endpoint (95% CI for the treatment difference [fondaparinux sodium-enoxaparin sodium] for VTE rates: -0.2%; 1.7%).
14.7 Treatment of Pulmonary Embolism
In a randomized, open-label, clinical trial in patients with a confirmed diagnosis of acute symptomatic PE, with or without DVT, ARIXTRA 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), or 10 mg (body weight >100 kg) SC once daily (ARIXTRA treatment regimen) was compared to heparin IV bolus (5,000 USP units) followed by a continuous IV infusion adjusted to maintain 1.5 to 2.5 times aPTT control value. Patients with a PE requiring thrombolysis or surgical thrombectomy were excluded from the trial. All patients started study treatment in hospital. Approximately 15% of patients were discharged home from the hospital while receiving ARIXTRA therapy. A total of 2,213 patients were randomized and 2,184 were treated. Patients ranged in age from 18 to 97 years (mean age 62 years) with 44% men and 56% women. Patients were 94% Caucasian, 5% black, and 1% other races. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. For both groups, treatment continued for at least 5 days with a treatment duration range 7 ± 2 days, and both treatment groups received vitamin K antagonist therapy initiated within 72 hours after the first study drug administration and continued for 90 ± 7 days, with regular dose adjustments to achieve an INR of 2 to 3. The primary efficacy endpoint was confirmed, symptomatic, recurrent VTE reported up to Day 97. The efficacy data are provided in Table 13.
Table 13. Efficacy of ARIXTRA in the Treatment of Pulmonary Embolism (All Randomized) - * VTE was a composite of symptomatic recurrent non-fatal VTE or fatal PE reported up to Day 97. The 95% confidence interval for the treatment difference for total VTE was: (‑3.0% to 0.5%).
Endpoint
ARIXTRA
5, 7.5, or 10 mg SC once daily
N = 1,103
Heparin
aPTT adjusted IV
N = 1,110
n
% (95% CI)
n
% (95% CI)
Total VTE*
42
3.8% (2.8, 5.1)
56
5.0% (3.8, 6.5)
DVT only
12
1.1% (0.6, 1.9)
17
1.5% (0.9, 2.4)
Non-fatal PE
14
1.3% (0.7, 2.1)
24
2.2% (1.4, 3.2)
Fatal PE
16
1.5% (0.8, 2.3)
15
1.4% (0.8, 2.2)
During the initial treatment period, 12 (1.1%) of patients treated with fondaparinux sodium and 19 (1.7%) of patients treated with heparin had a VTE endpoint (95% CI for the treatment difference [fondaparinux sodium-heparin] for VTE rates: -1.6%; 0.4%).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
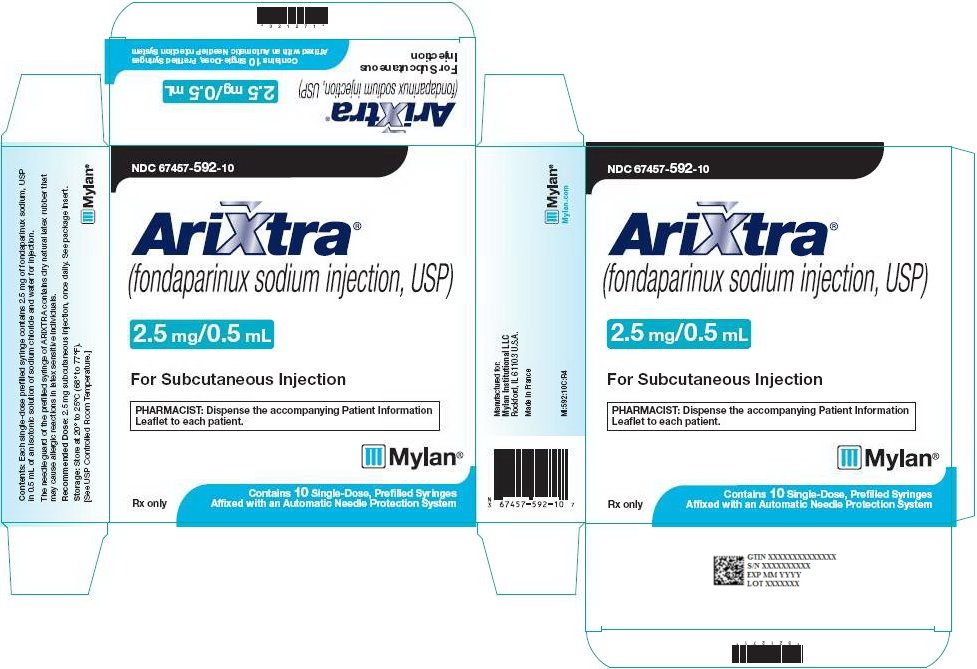
ARIXTRA (fondaparinux sodium injection, USP) is available in the following strengths:
2.5 mg ARIXTRA in 0.5 mL single-dose prefilled syringe, affixed with a 27-gauge x ½‑inch needle and an automatic needle protection system with white plunger rod.
NDC: 67457-592-10
10 Single Unit Syringes
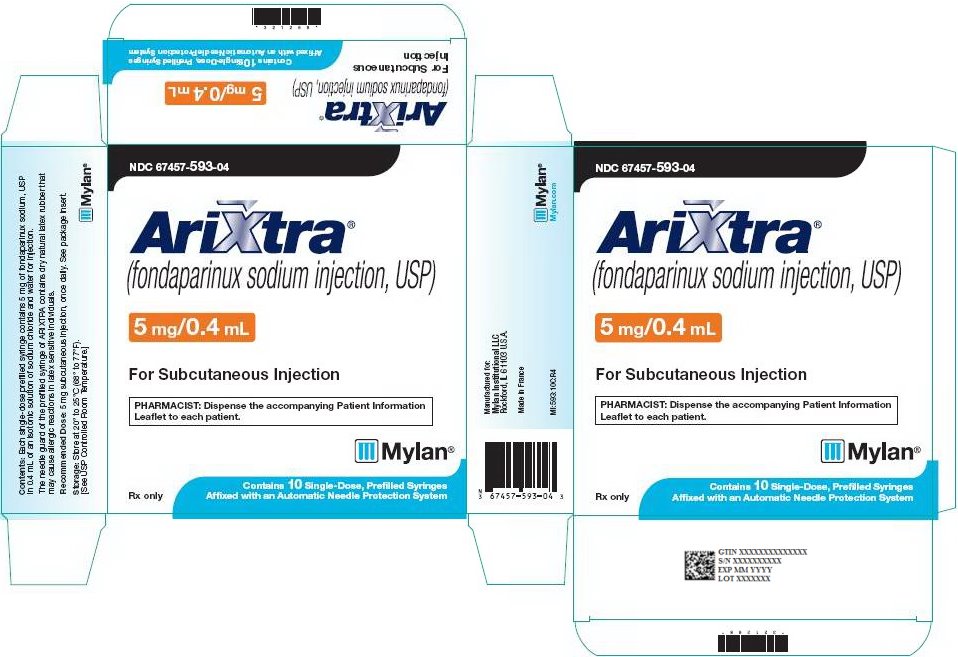
5 mg ARIXTRA in 0.4 mL single-dose prefilled syringe, affixed with a 27-gauge x ½‑inch needle and an automatic needle protection system with white plunger rod.
NDC: 67457-593-04
10 Single Unit Syringes
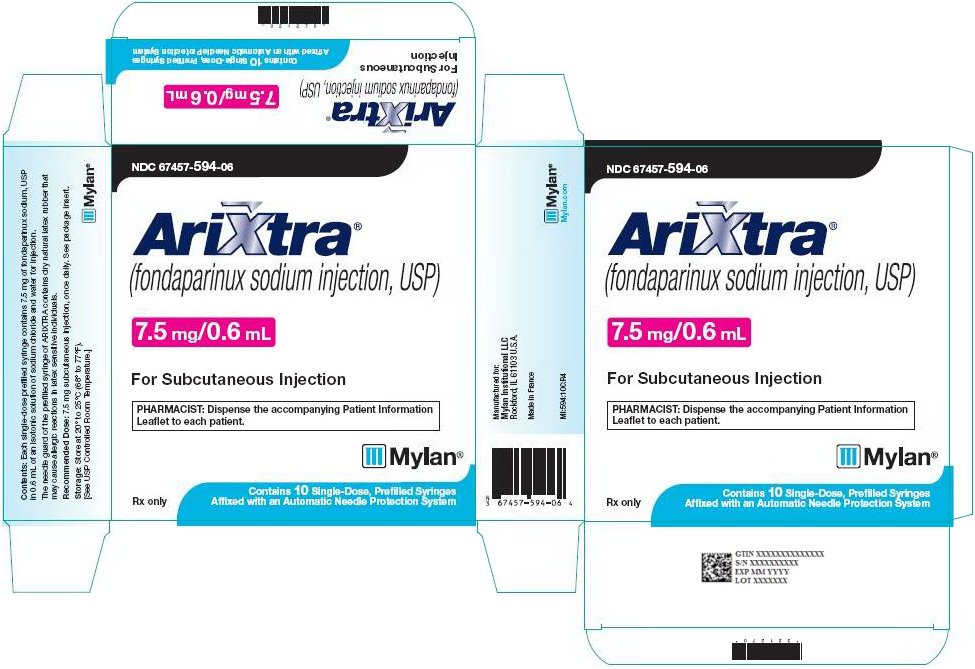
7.5 mg ARIXTRA in 0.6 mL single-dose prefilled syringe, affixed with a 27-gauge x ½‑inch needle and an automatic needle protection system with white plunger rod.
NDC: 67457-594-06
10 Single Unit Syringes
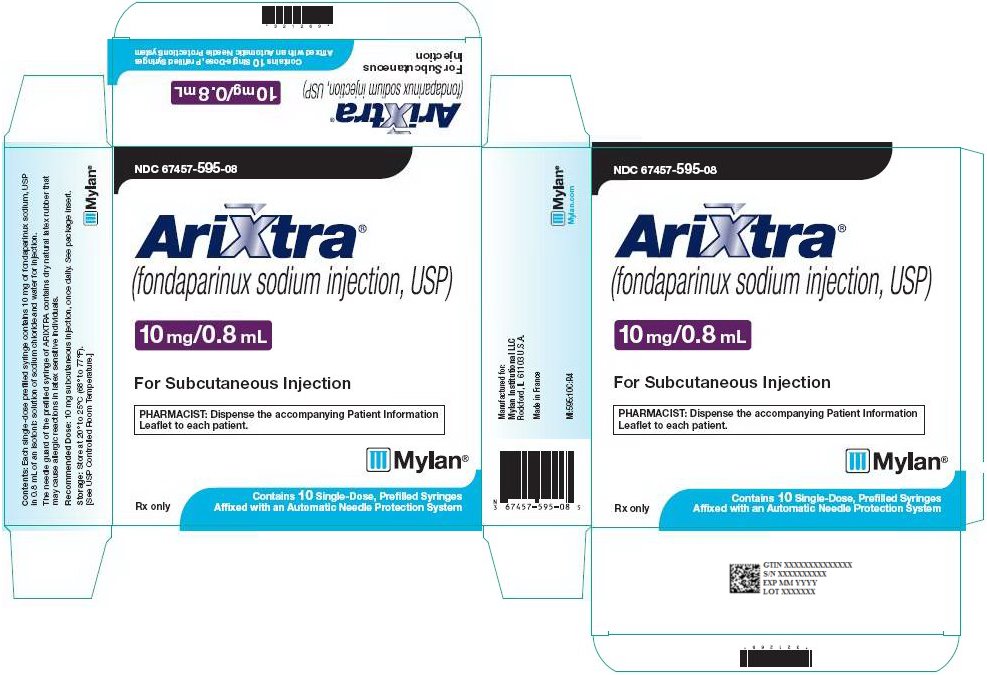
10 mg ARIXTRA in 0.8 mL single-dose prefilled syringe, affixed with a 27-gauge x ½‑inch needle and an automatic needle protection system with white plunger rod.
NDC: 67457-595-08
10 Single Unit Syringes
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Discard unused portion.
PHARMACIST: Dispense a Patient Information Leaflet with each prescription.
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (17.2)
17.1 Patient Advice
If the patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDS, platelet inhibitors, or other anticoagulants, they should be informed to watch for signs and symptoms of spinal or epidural hematomas, such as back pain, tingling, numbness (especially in the lower limbs), muscular weakness, and stool or urine incontinence. If any of these symptoms occur, the patients should contact his or her physician immediately.
The use of aspirin and other NSAIDS may enhance the risk of hemorrhage. Their use should be discontinued prior to ARIXTRA therapy whenever possible; if co-administration is essential, the patient’s clinical and laboratory status should be closely monitored [see Drug Interactions (7)].
If patients must self-administer ARIXTRA (e.g., if ARIXTRA is used at home), they should be advised of the following:
- ARIXTRA should be given by subcutaneous injection. Patients must be instructed in the proper technique for administration.
- The most important risk with ARIXTRA administration is bleeding. Patients should be counseled on signs and symptoms of possible bleeding.
- It may take them longer than usual to stop bleeding.
- They may bruise and/or bleed more easily when they are treated with ARIXTRA.
- They should report any unusual bleeding, bruising, or signs of thrombocytopenia (such as a rash of dark red spots under the skin) to their physician [see Warnings and Precautions (5.2, 5.5)].
- To tell their physicians and dentists they are taking ARIXTRA and/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is taken [see Warnings and Precautions (5.2)].
- To tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as aspirin or other NSAIDs [see Drug Interactions (7)].
Keep out of the reach of children.
-
PATIENT INFORMATION
ARIXTRA® (Ah-RIX-trah)
(fondaparinux sodium injection)
solution, for subcutaneous injectionWhat is the most important information I should know about ARIXTRA?
ARIXTRA may cause serious side effects, including:
-
Spinal or epidural blood clots (hematoma). People who take a blood thinner medicine (anticoagulant) like ARIXTRA, and have medicine injected into their spinal and epidural area, or have a spinal puncture have a risk of forming a blood clot that can cause long-term or permanent loss of the ability to move (paralysis). Your risk of developing a spinal or epidural blood clot is higher if:
- o a thin tube called an epidural catheter is placed in your back to give you certain medicine
- o you take NSAIDs or a medicine to prevent blood from clotting
- o you have a history of difficult or repeated epidural or spinal punctures
- o you have a history of problems with your spine or have had surgery on your spine
- If you take ARIXTRA and receive spinal anesthesia or have a spinal puncture, your doctor should watch you closely for symptoms of spinal or epidural blood clots. Tell your doctor right away if you have back pain, tingling, numbness, muscle weakness (especially in your legs and feet), loss of control of the bowels or bladder (incontinence).
-
Because the risk of bleeding may be higher, tell your doctor before taking ARIXTRA if you:
- o are also taking certain other medicines that affect blood clotting such as aspirin, an NSAID (for example, ibuprofen or naproxen), clopidogrel, or warfarin sodium
- o have bleeding problems
- o had problems in the past with pain medication given through the spine
- o have had surgery to your spine
- o have a spinal deformity
What is ARIXTRA?
ARIXTRA is a prescription medicine that is used to:
- help prevent blood clots from forming in people who have had certain surgeries of the hip, knee, or the stomach area (abdominal surgery)
- treat people who have blood clots in their legs or blood clots that travel to their lungs, along with the blood thinner medicine warfarin.
It is not known if ARIXTRA is safe and effective for use in children younger than 18 years of age.
Who should not take ARIXTRA?
Do not take ARIXTRA if you:
- have certain kidney problems
- have active bleeding problems
- have an infection in your heart
- have low platelet counts and if you test positive for a certain antibody while you are taking ARIXTRA
- weigh less than 110 pounds (50 kg) to prevent blood clots from surgery. See, “What are the possible side effects of ARIXTRA?”
- had a serious allergic reaction to ARIXTRA
What should I tell my doctor before taking ARIXTRA?
Before taking ARIXTRA, tell your doctor about all of your medical conditions, including if you:
- have had any bleeding problems (such as stomach ulcers)
- have had a stroke
- have had recent surgeries, including eye surgery
- have diabetic eye disease
- have kidney or liver problems
- have uncontrolled high blood pressure
- have a latex allergy. The packaging (needle guard) for ARIXTRA contains dry natural latex rubber.
- are pregnant or plan to become pregnant. ARIXTRA may harm your unborn baby. If you are pregnant, talk to your doctor about the best way for you to prevent or treat blood clots.
- are breastfeeding or plan to breastfeed. It is not known if ARIXTRA passes into breast milk. You and your doctor should decide if you will breastfeed during treatment with ARIXTRA.
Tell your doctor about all the medicines you take including prescriptions and over-the-counter medicines, vitamins, and herbal supplements. Some medicines can increase your risk of bleeding.
See “What is the most important information I should know about ARIXTRA?” Do not start taking any new medicines without first talking to your doctor.
Tell all your doctors and dentist that you take ARIXTRA, especially if you need to have any kind of surgery or a dental procedure. Keep a list of your medicines and show it to all your doctors and pharmacist before you start a new medicine.
How should I take ARIXTRA?
- See the detailed Instructions for Use that comes with ARIXTRA for information about how to give an ARIXTRA injection.
- If your doctor tells you that you may give yourself injections of ARIXTRA at home, you will be shown how to give the injections first before you do them on your own.
- Take ARIXTRA exactly as your doctor tells you to.
- ARIXTRA is given by injection under the skin (subcutaneous injection).
- If you miss a dose of ARIXTRA, take your dose as soon as you remember. Do not take 2 doses at the same time.
- If you take too much ARIXTRA, call your doctor right away.
What are possible side effects of ARIXTRA?
ARIXTRA can cause serious side effects. See “What is the most important information I should know about ARIXTRA?”
-
Severe bleeding. Certain conditions can increase your risk for severe bleeding, including:
- o some bleeding problems
- o some gastrointestinal problems including ulcers
- o some types of strokes
- o uncontrolled high blood pressure
- o diabetic eye disease
- o soon after brain, spine, or eye surgery
- Certain kidney problems can also increase your risk of bleeding with ARIXTRA. Your doctor may check your kidney function during your treatment with ARIXTRA.
- Increased bleeding risk in people undergoing certain surgeries who weigh less than 110 pounds (50 kg).
-
Low blood platelets (thrombocytopenia). Low blood platelets can happen when you take ARIXTRA. Platelets are blood cells that help your blood to clot normally. Your doctor may check your platelet counts during your treatment with ARIXTRA.
You may bruise or bleed more easily during your treatment with ARIXTRA, and it may take longer than usual for bleeding to stop. Tell your doctor if you have any signs or symptoms of bleeding, bruising or rash of dark red spots under the skin during your treatment with ARIXTRA.
Tell your doctor if you have any bleeding, bruising, or a rash of dark spots under the skin (thrombocytopenia).
The most common side effects of ARIXTRA include:
- bleeding problems
- bleeding, rash, and itching at the injection site (injection site reactions)
- sleep problems (insomnia)
- low red blood cell count (anemia)
- increased wound drainage
- low potassium in your blood (hypokalemia)
- dizziness
- purplish spots on skin (purpura)
- low blood pressure (hypotension)
- confusion
- fluid-filled blisters (bullous eruption)
- blood clots (hematoma)
- severe bleeding after surgery (post-operative hemorrhage)
These are not all the possible side effects of ARIXTRA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ARIXTRA?
- Store ARIXTRA at 68° to 77°F (20° to 25°C).
- Safely throw away ARIXTRA that is out of date or no longer needed.
Keep ARIXTRA and all medicines out of the reach of children.
General information about the safe and effective use of ARIXTRA
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use ARIXTRA for a condition for which it was not prescribed. Do not give ARIXTRA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about ARIXTRA that is written for healthcare professionals.
What are the ingredients in ARIXTRA?
Active ingredient: fondaparinux sodium
Inactive ingredients: sodium chloride and water for injection
For more information about ARIXTRA, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 12/2019
INSTRUCTIONS FOR USE
ARIXTRA® (Ah-RIX-trah)
(fondaparinux sodium injection)
solution, for subcutaneous useBe sure that you read, understand, and follow the step-by-step Instructions for Use, before you try to give yourself an injection of ARIXTRA for the first time and each time you get a new prescription. There may be new information. Talk to your doctor or pharmacist if you have any questions.
Do not use ARIXTRA if:
- the solution appears discolored (the solution should normally appear clear)
- you see any particles in the solution
- the syringe is damaged
How should I give an injection of ARIXTRA?
ARIXTRA is injected into a skin fold of the lower stomach area (abdomen). Do not inject ARIXTRA into muscle. Usually a doctor or nurse will give this injection to you. In some cases you may be taught how to do this yourself.
Instructions for self-administration
The different parts of ARIXTRA safety syringe are:
1. Rigid needle guard

2. Plunger
3. Finger-grip
4. Security sleeve
Syringe BEFORE USE

Syringe AFTER USE

1. Wash your hands well with soap and water, rinse, and towel dry.
2. Sit or lie down in a comfortable position. Choose a spot on the lower stomach area (abdomen), at least 2 inches below your belly button (Figure A). Change (alternate) between using the left and right side of the lower abdomen for each injection. If you have any questions talk to your nurse or doctor.
3. Clean the injection area with an alcohol swab.
4. Remove the needle guard, by first twisting it and then pulling it in a straight line away from the body of the syringe (Figure B). Discard (throw away) the needle guard.
-
- Do not touch the needle or let it come in contact with any surface before the injection. A small air bubble in the syringe is normal.
- To be sure that you do not lose any medicine from the syringe, do not try to remove air bubbles from the syringe before giving the injection.
5. Gently pinch the skin that has been cleaned to make a fold. Hold the fold between the thumb and the forefinger of one hand during the entire injection (Figure C).
6. Hold the syringe firmly in your other hand using the finger grip. Insert the full length of the needle directly up and down (at an angle of 90°) into the skin fold (Figure D).
7. Inject all of the medicine in the syringe by pressing down on the plunger as far as it goes. This will activate the automatic needle protection system (Figure E).
8. Release the plunger. The needle will withdraw automatically from the skin, and pull back (retract) into the security sleeve where it will be locked (Figure F). Throw away the used ARIXTRA syringe. See, “Disposing of used ARIXTRA needles and syringes” below.
Disposing of used ARIXTRA needles and syringes:
- Put your used ARIXTRA needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
-
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o made of a heavy-duty plastic,
- o can be closed with a tight-fitting, puncture- resistant lid, without sharps being able to come out,
- o upright and stable during use,
- o leak- resistant, and
- o properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration Revised: 12/2019
Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.Manufactured by:
Aspen Notre Dame de Bondeville
Notre Dame de Bondeville, FrancePL:MI:ARXTIJ:R5p
-
Spinal or epidural blood clots (hematoma). People who take a blood thinner medicine (anticoagulant) like ARIXTRA, and have medicine injected into their spinal and epidural area, or have a spinal puncture have a risk of forming a blood clot that can cause long-term or permanent loss of the ability to move (paralysis). Your risk of developing a spinal or epidural blood clot is higher if:
-
PRINCIPAL DISPLAY PANEL – 2.5 mg/0.5 mL
NDC: 67457-592-10
Arixtra®
(fondaparinux sodium injection, USP)2.5 mg/0.5 mL
For Subcutaneous Injection
PHARMACIST: Dispense the accompanying Patient Information
Leaflet to each patient.Rx only
Contains 10 Single-Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection SystemContents: Each single-dose prefilled syringe contains 2.5 mg of fondaparinux sodium, USP
in 0.5 mL of an isotonic solution of sodium chloride and water for injection.The needle guard of the prefilled syringe of ARIXTRA contains dry natural latex rubber that
may cause allergic reactions in latex sensitive individuals.Recommended Dose: 2.5 mg subcutaneous injection, once daily. See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.Made in France
MI:592:10C:R4
Mylan.com
-
PRINCIPAL DISPLAY PANEL – 5 mg/0.4 mL
NDC: 67457-593-04
Arixtra®
(fondaparinux sodium injection, USP)5 mg/0.4 mL
For Subcutaneous Injection
PHARMACIST: Dispense the accompanying Patient Information
Leaflet to each patient.Rx only
Contains 10 Single-Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection SystemContents: Each single-dose prefilled syringe contains 5 mg of fondaparinux sodium, USP
in 0.4 mL of an isotonic solution of sodium chloride and water for injection.The needle guard of the prefilled syringe of ARIXTRA contains dry natural latex rubber that
may cause allergic reactions in latex sensitive individuals.Recommended Dose: 5 mg subcutaneous injection, once daily. See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.Made in France
MI:593:10C:R4
Mylan.com
-
PRINCIPAL DISPLAY PANEL – 7.5 mg/0.6 mL
NDC: 67457-594-06
Arixtra®
(fondaparinux sodium injection, USP)7.5 mg/0.6 mL
For Subcutaneous Injection
PHARMACIST: Dispense the accompanying Patient Information
Leaflet to each patient.Rx only
Contains 10 Single-Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection SystemContents: Each single-dose prefilled syringe contains 7.5 mg of fondaparinux sodium, USP
in 0.6 mL of an isotonic solution of sodium chloride and water for injection.The needle guard of the prefilled syringe of ARIXTRA contains dry natural latex rubber that
may cause allergic reactions in latex sensitive individuals.Recommended Dose: 7.5 mg subcutaneous injection, once daily. See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.Made in France
MI:594:10C:R4
Mylan.com
-
PRINCIPAL DISPLAY PANEL – 10 mg/0.8 mL
NDC 67457-595-08
Arixtra®
(fondaparinux sodium injection, USP)10 mg/0.8 mL
For Subcutaneous Injection
PHARMACIST: Dispense the accompanying Patient Information
Leaflet to each patient.Rx only
Contains 10 Single-Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection SystemContents: Each single-dose prefilled syringe contains 10 mg of fondaparinux sodium, USP
in 0.8 mL of an isotonic solution of sodium chloride and water for injection.The needle guard of the prefilled syringe of ARIXTRA contains dry natural latex rubber that
may cause allergic reactions in latex sensitive individuals.Recommended Dose: 10 mg subcutaneous injection, once daily. See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.Made in France
MI:595:10C:R4
Mylan.com
-
INGREDIENTS AND APPEARANCE
ARIXTRA
fondaparinux sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67457-592 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FONDAPARINUX SODIUM (UNII: X0Q6N9USOZ) (FONDAPARINUX - UNII:J177FOW5JL) FONDAPARINUX SODIUM 2.5 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67457-592-10 10 in 1 CARTON 05/06/2015 1 NDC: 67457-592-00 0.5 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021345 05/06/2015 ARIXTRA
fondaparinux sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67457-593 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FONDAPARINUX SODIUM (UNII: X0Q6N9USOZ) (FONDAPARINUX - UNII:J177FOW5JL) FONDAPARINUX SODIUM 5 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67457-593-04 10 in 1 CARTON 08/07/2015 1 NDC: 67457-593-00 0.4 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021345 08/07/2015 ARIXTRA
fondaparinux sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67457-594 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FONDAPARINUX SODIUM (UNII: X0Q6N9USOZ) (FONDAPARINUX - UNII:J177FOW5JL) FONDAPARINUX SODIUM 7.5 mg in 0.6 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67457-594-06 10 in 1 CARTON 02/11/2016 1 NDC: 67457-594-00 0.6 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021345 02/11/2016 ARIXTRA
fondaparinux sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67457-595 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FONDAPARINUX SODIUM (UNII: X0Q6N9USOZ) (FONDAPARINUX - UNII:J177FOW5JL) FONDAPARINUX SODIUM 10 mg in 0.8 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67457-595-08 10 in 1 CARTON 11/13/2015 1 NDC: 67457-595-00 0.8 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021345 11/13/2015 Labeler - Mylan Institutional LLC (790384502)
Trademark Results [Arixtra]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ARIXTRA 76251411 2823751 Live/Registered |
MYLAN INSTITUTIONAL INC. 2001-05-04 |
 ARIXTRA 76251409 2725323 Live/Registered |
MYLAN INSTITUTIONAL INC. 2001-05-04 |
 ARIXTRA 75651561 2321564 Live/Registered |
MYLAN INSTITUTIONAL INC. 1999-03-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


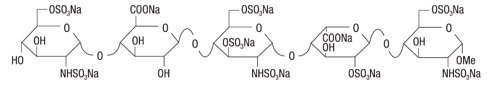
 Figure A.
Figure A. Figure B.
Figure B.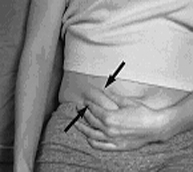 Figure C.
Figure C.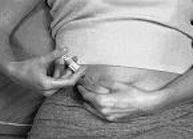 Figure D.
Figure D.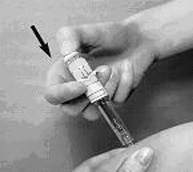 Figure E.
Figure E.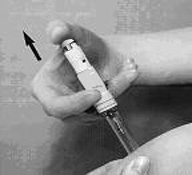 Figure F.
Figure F.