BLANC DIVA GLEAM COVERAGE CUSHION White by AHNNK GLOBAL Co., Ltd. / OXYGEN DEVELOPMENT, L.L.C.
BLANC DIVA GLEAM COVERAGE CUSHION White by
Drug Labeling and Warnings
BLANC DIVA GLEAM COVERAGE CUSHION White by is a Otc medication manufactured, distributed, or labeled by AHNNK GLOBAL Co., Ltd., OXYGEN DEVELOPMENT, L.L.C.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
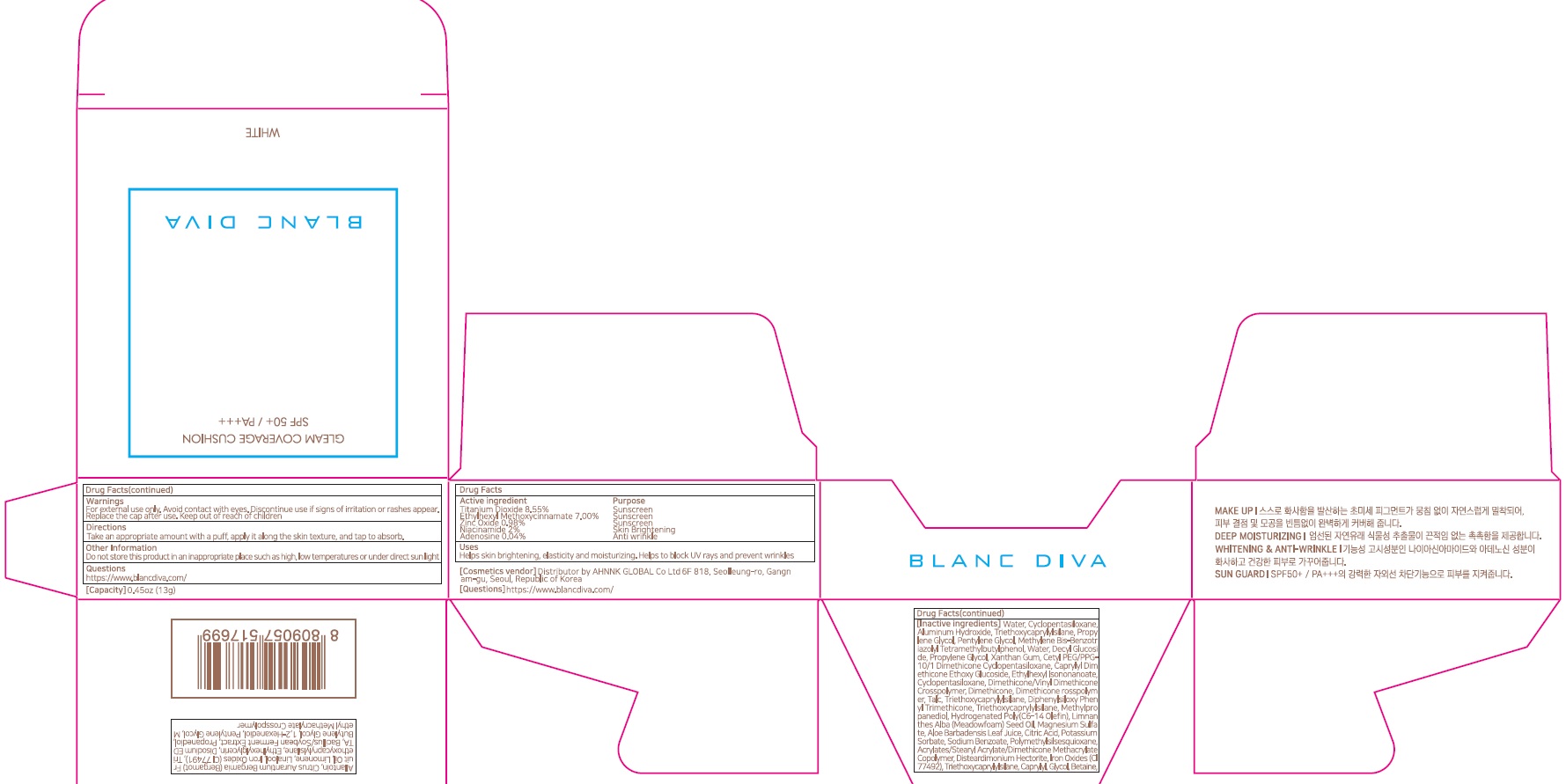
BLANC DIVA GLEAM COVERAGE CUSHION WHITE- titanium dioxide, ethylhexyl methoxycinnamate, zinc oxide, niacinamide, adenosine powder
AHNNK GLOBAL Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ACTIVE INGREDIENTS
Titanium Dioxide 8.55%
Ethylhexyl Methoxycinnamate 7.00%
Zinc Oxide 0.98%
Niacinamide 2%
Adenosine 0.04%
INACTIVE INGREDIENTS
Water, Cyclopentasiloxane, Aluminum Hydroxide, Triethoxycaprylylsilane, Propylene Glycol, Pentylene Glycol, Methylene Bis-Benzotriazolyl Tetramethylbutylphenol, Water, Decyl Glucoside, Propylene Glycol, Xanthan Gum, Cetyl PEG/PPG-10/1 Dimethicone Cyclopentasiloxane, Caprylyl Dimethicone Ethoxy Glucoside, Ethylhexyl Isononanoate, Cyclopentasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Dimethicone, Dimethicone rosspolymer, Talc, Triethoxycaprylylsilane, Diphenylsiloxy Phenyl Trimethicone, Triethoxycaprylylsilane , Methylpropanediol, Hydrogenated Poly(C6-14 Olefin), Limnanthes Alba (Meadowfoam) Seed Oil, Magnesium Sulfate, Aloe Barbadensis Leaf Juice,
Citric Acid,Potassium Sorbate, Sodium Benzoate, Polymethylsilsesquioxane, Acrylates/Stearyl Acrylate/Dimethicone Methacrylate Copolymer, Disteardimonium Hectorite, Iron Oxides (CI 77492), Triethoxycaprylylsilane, Caprylyl Glycol, Betaine, Allantoin, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Limonene, Linalool, Iron Oxides (CI 77491), Triethoxycaprylylsilane, Ethylhexylglycerin, Disodium EDTA, Bacillus/Soybean Ferment Extract, Propanediol, Butylene Glycol, 1,2-Hexanediol, Pentylene Glycol, Methyl Methacrylate Crosspolymer
WARNINGS
For external use only
Avoid contact with eyes.
Discontinue use if signs of irritation or rashes appear.
Replace the cap after use.
Refrain from using on wounded areas.
Store away from direct sunlight
Keep out of reach of children
Uses
■ Helps skin brightening, elasticity and moisturizing
■ Helps to block UV rays and prevent wrinkles
Directions
■ Take an appropriate amount with a puff, apply it along the skin texture, and tap to absorb.
| BLANC DIVA GLEAM COVERAGE CUSHION WHITE
titanium dioxide, ethylhexyl methoxycinnamate, zinc oxide, niacinamide, adenosine powder |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - AHNNK GLOBAL Co., Ltd. (695794480) |
| Registrant - AHNNK GLOBAL Co., Ltd. (695794480) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| OXYGEN DEVELOPMENT, L.L.C. | 137098492 | manufacture(82485-030) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.