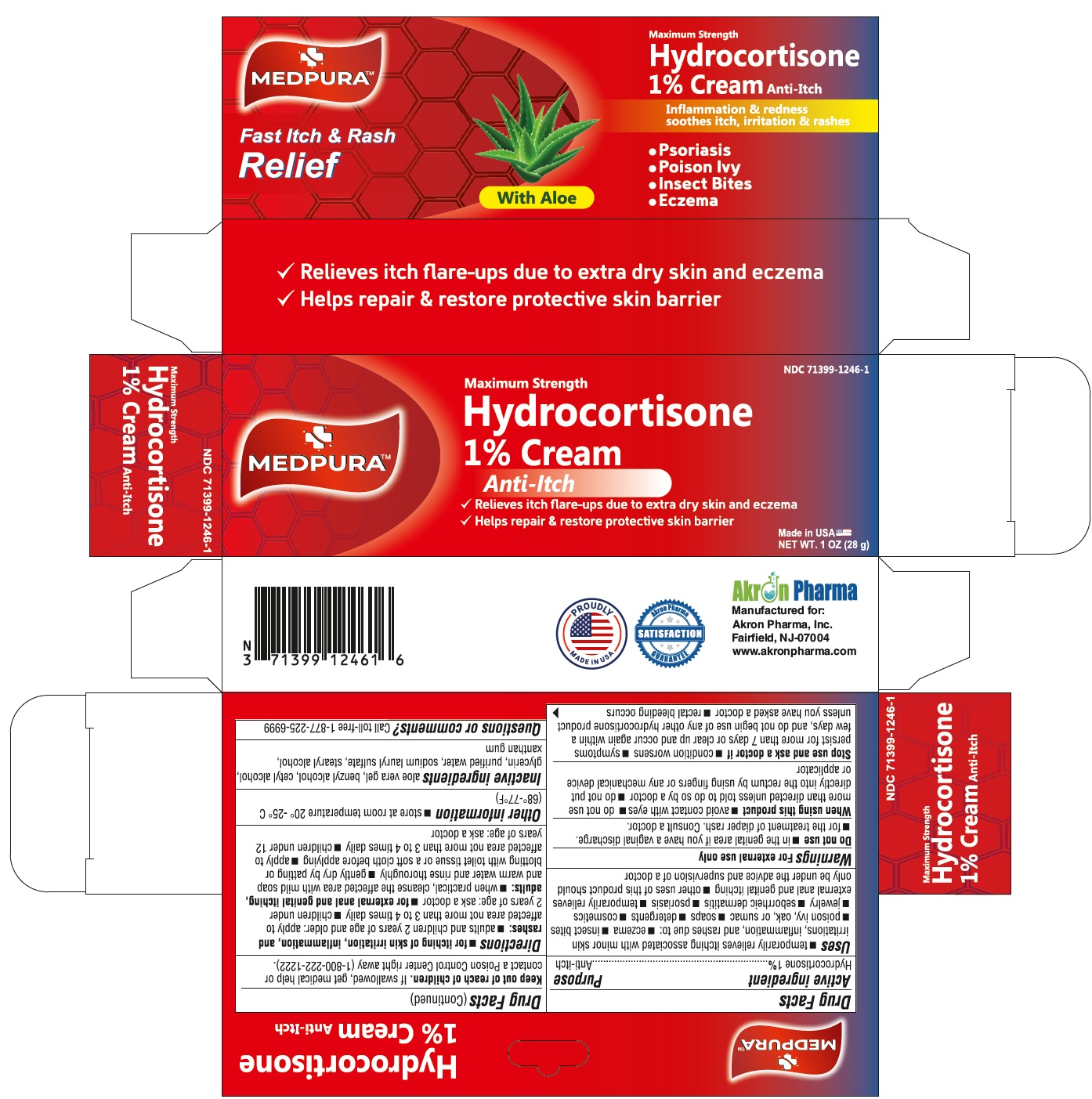
Hydrocortisone Anti-Itch Cream 1%Drug Facts
Hydrocortisone by
Drug Labeling and Warnings
Hydrocortisone by is a Otc medication manufactured, distributed, or labeled by AKRON PHARMA INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HYDROCORTISONE- hydrocortisone cream
AKRON PHARMA INC
----------
Hydrocortisone Anti-Itch Cream 1%
Drug Facts
Use
temporarily relieves itching associated with minor skin irritations, inflammation and rashes due to:
- eczema
- insect bites
- poison ivy or sumac
- soaps
- detergents
- cosmetics
- jewelry,
- seborrheic dermatitis
- poison oak
- psoriasis
- temporarily relieves external anal and genital itching
- Other uses of this product should be undertaken only under the advice and supervision of a doctor.
Do not use
- in the genital area if you have a vaginal discharge.
- for the treatment of diaper rash, Consult a doctor.
When using this product
- avoid contact with eyes
- do not use more thatn directed unless told to do so by a doctor
- do not put directly into the rectumby using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- Condition worsens, symptoms persist for more than 7 days or clear up and occur again within a few days. Discontinue use of this product and do not begin use of any other hydrocortisone product unless you have consulted a doctor.
- rectal bleeding occurs.
Keep out of Reach of Children
If swallowed, get medical help or contact Poison Control Center right away (1-800-222-1222).
Directions
for itching of skin irritation, inflammation, and rashes:
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
for external anal and genital itching, adults:
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
| HYDROCORTISONE
hydrocortisone cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - AKRON PHARMA INC (067878881) |
Revised: 11/2023
Document Id: 95eea1cc-bf73-4fdc-9532-3fe41547b29b
Set id: d3dd60a8-b66f-4d32-8e79-7d888d5b6493
Version: 3
Effective Time: 20231122
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.