AMIODARONE HYDROCHLORIDE tablet
Amiodarone Hydrochloride by
Drug Labeling and Warnings
Amiodarone Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by NCS HealthCare of KY, LLC dba Vangard Labs. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Amiodarone hydrochloride is a member of a class of antiarrhythmic drugs with predominantly Class III (Vaughan Williams’ classification) effects, available for oral administration as white to off-white, scored tablets containing 200 mg of amiodarone hydrochloride USP. The inactive ingredients present are colloidal silicon dioxide, lactose monohydrate, magnesium stearate, povidone, and sodium starch glycolate. Amiodarone hydrochloride is a benzofuran derivative: 2-butyl-3-benzofuranyl 4-[2-(diethylamino)-ethoxy]-3,5-diiodophenyl ketone hydrochloride.
The structural formula is as follows:
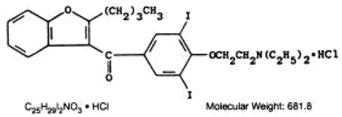
Amiodarone hydrochloride USP is a white or almost white, fine crystalline powder. It is slightly soluble in water, soluble in alcohol, and freely soluble in chloroform. It contains 37.3% iodine by weight.
-
CLINICAL PHARMACOLOGY
Electrophysiology/Mechanisms of Action
In animals, amiodarone hydrochloride is effective in the prevention or suppression of experimentally induced arrhythmias. The antiarrhythmic effect of amiodarone hydrochloride may be due to at least two major properties:
- a prolongation of the myocardial cell-action potential duration and refractory period and
- non-competitive antagonism of α- and β-adrenoceptors.
Amiodarone hydrochloride prolongs the duration of the action potential of all cardiac fibers while causing minimal reduction of dV/dt (maximal upstroke velocity of the action potential). The refractory period is prolonged in all cardiac tissues. Amiodarone hydrochloride increases the cardiac refractory period without influencing resting membrane potential, except in automatic cells where the slope of the prepotential is reduced, generally reducing automaticity. These electrophysiologic effects are reflected in a decreased sinus rate of 15 to 20%, increased PR and QT intervals of about 10%, the development of U-waves, and changes in T-wave contour. These changes should not require discontinuation of amiodarone hydrochloride as they are evidence of its pharmacological action, although amiodarone hydrochloride can cause marked sinus bradycardia or sinus arrest and heart block. On rare occasions, QT prolongation has been associated with worsening of arrhythmia (see “WARNINGS”).
Hemodynamics
In animal studies and after intravenous administration in man, amiodarone hydrochloride relaxes vascular smooth muscle, reduces peripheral vascular resistance (afterload), and slightly increases cardiac index. After oral dosing, however, amiodarone hydrochloride produces no significant change in left ventricular ejection fraction (LVEF), even in patients with depressed LVEF. After acute intravenous dosing in man, amiodarone hydrochloride may have a mild negative inotropic effect.
Pharmacokinetics
Following oral administration in man, amiodarone hydrochloride is slowly and variably absorbed. The bioavailability of amiodarone hydrochloride is approximately 50%, but has varied between 35 and 65% in various studies. Maximum plasma concentrations are attained 3 to 7 hours after a single dose. Despite this, the onset of action may occur in 2 to 3 days, but more commonly takes 1 to 3 weeks, even with loading doses. Plasma concentrations with chronic dosing at 100 to 600 mg/day are approximately dose proportional, with a mean 0.5 mg/L increase for each 100 mg/day. These means, however, include considerable individual variability. Food increases the rate and extent of absorption of amiodarone hydrochloride. The effects of food upon the bioavailability of amiodarone hydrochloride have been studied in 30 healthy subjects who received a single 600 mg dose immediately after consuming a high-fat meal and following an overnight fast. The area under the plasma concentration-time curve (AUC) and the peak plasma concentration (Cmax) of amiodarone increased by 2.3 (range 1.7 to 3.6) and 3.8 (range 2.7 to 4.4) times, respectively, in the presence of food. Food also increased the rate of absorption of amiodarone, decreasing the time to peak plasma concentration (Tmax) by 37%. The mean AUC and mean Cmaxof the major metabolite of amiodarone, desethylamiodarone (DEA) increased by 55% (range 58 to 101%) and 32% (range 4 to 84%), respectively, but there was no change in the Tmax in the presence of food.
Amiodarone hydrochloride has a very large but variable volume of distribution, averaging about 60 L/kg, because of extensive accumulation in various sites, especially adipose tissue and highly perfused organs, such as the liver, lung, and spleen. One major metabolite of amiodarone hydrochloride, DEA, has been identified in man; it accumulates to an even greater extent in almost all tissues. No data are available on the activity of DEA in humans, but in animals, it has significant electrophysiologic and antiarrhythmic effects generally similar to amiodarone itself. DEA’s precise role and contribution to the antiarrhythmic activity of oral amiodarone are not certain. The development of maximal ventricular Class III effects after oral amiodarone hydrochloride administration in humans correlates more closely with DEA accumulation over time than with amiodarone accumulation.
Amiodarone is metabolized to DEA by the cytochrome P450 (CYP) enzyme group, specifically CYP3A and CYP2C8. The CYP3A isoenzyme is present in both the liver and intestines. In vitro, amiodarone and DEA, exhibit a potential to inhibit CYP2C9, CYP2C19, CYP2D6, CYP3A, CYP2A6, CYP2B6 and CYP2C8. Amiodarone and DEA have also a potential to inhibit some transporters such as P-glycoprotein and organic cation transporter (OCT2).
Amiodarone is eliminated primarily by hepatic metabolism and biliary excretion and there is negligible excretion of amiodarone or DEA in urine. Neither amiodarone nor DEA is dialyzable.
In clinical studies of 2 to 7 days, clearance of amiodarone after intravenous administration in patients with VT and VF ranged between 220 and 440 mL/hr/kg. Age, sex, renal disease, and hepatic disease (cirrhosis) do not have marked effects on the disposition of amiodarone or DEA. Renal impairment does not influence the pharmacokinetics of amiodarone. After a single dose of intravenous amiodarone in cirrhotic patients, significantly lower Cmax and average concentration values are seen for DEA, but mean amiodarone levels are unchanged. Normal subjects over 65 years of age show lower clearances (about 100 mL/hr/kg) than younger subjects (about 150 mL/hr/kg) and an increase in t½ from about 20 to 47 days. In patients with severe left ventricular dysfunction, the pharmacokinetics of amiodarone are not significantly altered but the terminal disposition t½ of DEA is prolonged. Although no dosage adjustment for patients with renal, hepatic, or cardiac abnormalities has been defined during chronic treatment with amiodarone hydrochloride, close clinical monitoring is prudent for elderly patients and those with severe left ventricular dysfunction.
Following single dose administration in 12 healthy subjects, amiodarone hydrochloride exhibited multi-compartmental pharmacokinetics with a mean apparent plasma terminal elimination half-life of 58 days (range 15 to 142 days) for amiodarone and 36 days (range 14 to 75 days) for the active metabolite (DEA). In patients, following discontinuation of chronic oral therapy, amiodarone hydrochloride has been shown to have a biphasic elimination with an initial one-half reduction of plasma levels after 2.5 to 10 days. A much slower terminal plasma-elimination phase shows a half-life of the parent compound ranging from 26 to 107 days, with a mean of approximately 53 days and most patients in the 40- to 55-day range. In the absence of a loading-dose period, steady-state plasma concentrations, at constant oral dosing, would therefore be reached between 130 and 535 days, with an average of 265 days. For the metabolite, the mean plasma-elimination half-life was approximately 61 days. These data probably reflect an initial elimination of drug from well-perfused tissue (the 2.5- to 10-day half-life phase), followed by a terminal phase representing extremely slow elimination from poorly perfused tissue compartments such as fat.
The considerable intersubject variation in both phases of elimination, as well as uncertainty as to what compartment is critical to drug effect, requires attention to individual responses once arrhythmia control is achieved with loading doses because the correct maintenance dose is determined, in part, by the elimination rates. Daily maintenance doses of amiodarone hydrochloride should be based on individual patient requirements (see “DOSAGE AND ADMINISTRATION”).
Amiodarone hydrochloride and its metabolite have a limited transplacental transfer of approximately 10 to 50%. The parent drug and its metabolite have been detected in breast milk.
Amiodarone hydrochloride is highly protein-bound (approximately 96%).
Although electrophysiologic effects, such as prolongation of QTc, can be seen within hours after a parenteral dose of amiodarone hydrochloride, effects on abnormal rhythms are not seen before 2 to 3 days and usually require 1 to 3 weeks, even when a loading dose is used. There may be a continued increase in effect for longer periods still. There is evidence that the time to effect is shorter when a loading-dose regimen is used.
Consistent with the slow rate of elimination, antiarrhythmic effects persist for weeks or months after amiodarone hydrochloride is discontinued, but the time of recurrence is variable and unpredictable. In general, when the drug is resumed after recurrence of the arrhythmia, control is established relatively rapidly compared to the initial response, presumably because tissue stores were not wholly depleted at the time of recurrence.Pharmacodynamics
There is no well-established relationship of plasma concentration to effectiveness, but it does appear that concentrations much below 1 mg/L are often ineffective and that levels above 2.5 mg/L are generally not needed. Within individuals dose reductions and ensuing decreased plasma concentrations can result in loss of arrhythmia control. Plasma-concentration measurements can be used to identify patients whose levels are unusually low, and who might benefit from a dose increase, or unusually high, and who might have dosage reduction in the hope of minimizing side effects. Some observations have suggested a plasma concentration, dose, or dose/duration relationship for side effects such as pulmonary fibrosis, liver-enzyme elevations, corneal deposits and facial pigmentation, peripheral neuropathy, gastrointestinal and central nervous system effects.
Monitoring Effectiveness
Predicting the effectiveness of any antiarrhythmic agent in long-term prevention of recurrent ventricular tachycardia and ventricular fibrillation is difficult and controversial, with highly qualified investigators recommending use of ambulatory monitoring, programmed electrical stimulation with various stimulation regimens, or a combination of these, to assess response. There is no present consensus on many aspects of how best to assess effectiveness, but there is a reasonable consensus on some aspects:
- If a patient with a history of cardiac arrest does not manifest a hemodynamically unstable arrhythmia during electrocardiographic monitoring prior to treatment, assessment of the effectiveness of amiodarone hydrochloride requires some provocative approach, either exercise or programmed electrical stimulation (PES).
- Whether provocation is also needed in patients who do manifest their life-threatening arrhythmia spontaneously is not settled, but there are reasons to consider PES or other provocation in such patients. In the fraction of patients whose PES-inducible arrhythmia can be made noninducible by amiodarone hydrochloride (a fraction that has varied widely in various series from less than 10% to almost 40%, perhaps due to different stimulation criteria), the prognosis has been almost uniformly excellent, with very low recurrence (ventricular tachycardia or sudden death) rates. More controversial is the meaning of continued inducibility. There has been an impression that continued inducibility in amiodarone hydrochloride patients may not foretell a poor prognosis but, in fact, many observers have found greater recurrence rates in patients who remain inducible than in those who do not. A number of criteria have been proposed, however, for identifying patients who remain inducible but who seem likely nonetheless to do well on amiodarone hydrochloride. These criteria include increased difficulty of induction (more stimuli or more rapid stimuli), which has been reported to predict a lower rate of recurrence, and ability to tolerate the induced ventricular tachycardia without severe symptoms, a finding that has been reported to correlate with better survival but not with lower recurrence rates. While these criteria require confirmation and further study in general, easier inducibility or poorer tolerance of the induced arrhythmia should suggest consideration of a need to revise treatment.
Several predictors of success not based on PES have also been suggested, including complete elimination of all nonsustained ventricular tachycardia on ambulatory monitoring and very low premature ventricular-beat rates (less than 1 VPB/1,000 normal beats).
While these issues remain unsettled for amiodarone hydrochloride, as for other agents, the prescriber of amiodarone hydrochloride should have access to (direct or through referral), and familiarity with, the full range of evaluatory procedures used in the care of patients with life-threatening arrhythmias.
It is difficult to describe the effectiveness rates of amiodarone hydrochloride, as these depend on the specific arrhythmia treated, the success criteria used, the underlying cardiac disease of the patient, the number of drugs tried before resorting to amiodarone hydrochloride, the duration of follow-up, the dose of amiodarone hydrochloride, the use of additional antiarrhythmic agents, and many other factors. As amiodarone hydrochloride has been studied principally in patients with refractory life-threatening ventricular arrhythmias, in whom drug therapy must be selected on the basis of response and cannot be assigned arbitrarily, randomized comparisons with other agents or placebo have not been possible. Reports of series of treated patients with a history of cardiac arrest and mean follow-up of one year or more have given mortality (due to arrhythmia) rates that were highly variable, ranging from less than 5% to over 30%, with most series in the range of 10 to 15%. Overall arrhythmia-recurrence rates (fatal and nonfatal) also were highly variable (and, as noted above, depended on response to PES and other measures), and depend on whether patients who do not seem to respond initially are included. In most cases, considering only patients who seemed to respond well enough to be placed on long-term treatment, recurrence rates have ranged from 20 to 40% in series with a mean follow-up of a year or more. -
INDICATIONS AND USAGE
Because of its life-threatening side effects and the substantial management difficulties associated with its use (see “WARNINGS” below), amiodarone hydrochloride tablets are indicated only for the treatment of the following documented, life-threatening recurrent ventricular arrhythmias when these have not responded to documented adequate doses of other available antiarrhythmics or when alternative agents could not be tolerated.
- Recurrent ventricular fibrillation.
- Recurrent hemodynamically unstable ventricular tachycardia.
As is the case for other antiarrhythmic agents, there is no evidence from controlled trials that the use of amiodarone hydrochloride tablets favorably affects survival.
Amiodarone hydrochloride tablets should be used only by physicians familiar with and with access to (directly or through referral) the use of all available modalities for treating recurrent life-threatening ventricular arrhythmias, and who have access to appropriate monitoring facilities, including in-hospital and ambulatory continuous electrocardiographic monitoring and electrophysiologic techniques. Because of the life-threatening nature of the arrhythmias treated, potential interactions with prior therapy, and potential exacerbation of the arrhythmia, initiation of therapy with amiodarone hydrochloride tablets should be carried out in the hospital. -
CONTRAINDICATIONS
Amiodarone hydrochloride tablets are contraindicated in patients with cardiogenic shock; severe sinus-node dysfunction, causing marked sinus bradycardia; second- or third-degree atrioventricular block; and when episodes of bradycardia have caused syncope (except when used in conjunction with a pacemaker).
Amiodarone hydrochloride tablets are contraindicated in patients with a known hypersensitivity to the drug or to any of its components, including iodine. -
WARNINGS
Amiodarone hydrochloride is intended for use only in patients with the indicated life-threatening arrhythmias because its use is accompanied by substantial toxicity.
Amiodarone hydrochloride has several potentially fatal toxicities, the most important of which is pulmonary toxicity (hypersensitivity pneumonitis or interstitial/alveolar pneumonitis) that has resulted in clinically manifest disease at rates as high as 10 to 17% in some series of patients with ventricular arrhythmias given doses around 400 mg/day, and as abnormal diffusion capacity without symptoms in a much higher percentage of patients. Pulmonary toxicity has been fatal about 10% of the time. Liver injury is common with amiodarone hydrochloride, but is usually mild and evidenced only by abnormal liver enzymes. Overt liver disease can occur, however, and has been fatal in a few cases. Like other antiarrhythmics, amiodarone hydrochloride can exacerbate the arrhythmia, e.g., by making the arrhythmia less well tolerated or more difficult to reverse. This has occurred in 2 to 5% of patients in various series, and significant heart block or sinus bradycardia has been seen in 2 to 5%. All of these events should be manageable in the proper clinical setting in most cases. Although the frequency of such proarrhythmic events does not appear greater with amiodarone hydrochloride than with many other agents used in this population, the effects are prolonged when they occur.
Even in patients at high risk of arrhythmic death, in whom the toxicity of amiodarone hydrochloride is an acceptable risk, amiodarone hydrochloride poses major management problems that could be life-threatening in a population at risk of sudden death, so that every effort should be made to utilize alternative agents first.
The difficulty of using amiodarone hydrochloride effectively and safely itself poses a significant risk to patients. Patients with the indicated arrhythmias must be hospitalized while the loading dose of amiodarone hydrochloride is given, and a response generally requires at least one week, usually two or more. Because absorption and elimination are variable, maintenance-dose selection is difficult, and it is not unusual to require dosage decrease or discontinuation of treatment. In a retrospective survey of 192 patients with ventricular tachyarrhythmias, 84 required dose reduction and 18 required at least temporary discontinuation because of adverse effects, and several series have reported 15 to 20% overall frequencies of discontinuation due to adverse reactions. The time at which a previously controlled life-threatening arrhythmia will recur after discontinuation or dose adjustment is unpredictable, ranging from weeks to months. The patient is obviously at great risk during this time and may need prolonged hospitalization. Attempts to substitute other antiarrhythmic agents when amiodarone hydrochloride must be stopped will be made difficult by the gradually, but unpredictably, changing amiodarone body burden. A similar problem exists when amiodarone hydrochloride is not effective; it still poses the risk of an interaction with whatever subsequent treatment is tried.Mortality
In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-centered, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had myocardial infarctions more than six days but less than two years previously, an excessive mortality or non-fatal cardiac arrest rate was seen in patients treated with encainide or flecainide (56/730) compared with that seen in patients assigned to matched placebo-treated groups (22/725). The average duration of treatment with encainide or flecainide in this study was ten months.
Amiodarone hydrochloride therapy was evaluated in two multi-centered, randomized, double-blind, placebo-controlled trials involving 1202 (Canadian Amiodarone Myocardial Infarction Arrhythmia Trial; CAMIAT) and 1486 (European Myocardial Infarction Amiodarone Trial; EMIAT) post-MI patients followed for up to 2 years. Patients in CAMIAT qualified with ventricular arrhythmias, and those randomized to amiodarone received weight- and response-adjusted doses of 200 to 400 mg/day. Patients in EMIAT qualified with ejection fraction <40%, and those randomized to amiodarone received fixed doses of 200 mg/day. Both studies had weeks-long loading dose schedules. Intent-to-treat all-cause mortality results were as follows:
Placebo Amiodarone Relative Risk N Deaths N Deaths 95%CI EMIAT
743
102
743
103
0.99
0.76 to 1.31
CAMIAT
596
68
606
57
0.88
0.58 to 1.16
These data are consistent with the results of a pooled analysis of smaller, controlled studies involving patients with structural heart disease (including myocardial infarction).
Pulmonary Toxicity
There have been postmarketing reports of acute-onset (days to weeks) pulmonary injury in patients treated with oral amiodarone hydrochloride with or without initial I.V. therapy. Findings have included pulmonary infiltrates and/or mass on X-ray, pulmonary alveolar hemorrhage, pleural effusion, bronchospasm, wheezing, fever, dyspnea, cough, hemoptysis, and hypoxia. Some cases have progressed to respiratory failure and/or death. Postmarketing reports describe cases of pulmonary toxicity in patients treated with low doses of amiodarone hydrochloride; however, reports suggest that the use of lower loading and maintenance doses of amiodarone hydrochloride are associated with a decreased incidence of amiodarone hydrochloride-induced pulmonary toxicity.
Amiodarone hydrochloride tablets may cause a clinical syndrome of cough and progressive dyspnea accompanied by functional, radiographic, gallium-scan, and pathological data consistent with pulmonary toxicity, the frequency of which varies from 2 to 7% in most published reports, but is as high as 10 to 17% in some reports. Therefore, when amiodarone hydrochloride therapy is initiated, a baseline chest X-ray and pulmonary-function tests, including diffusion capacity, should be performed. The patient should return for a history, physical exam, and chest X-ray every 3 to 6 months.
Pulmonary toxicity secondary to amiodarone hydrochloride seems to result from either indirect or direct toxicity as represented by hypersensitivity pneumonitis (including eosinophilic pneumonia) or interstitial/alveolar pneumonitis, respectively.
Patients with preexisting pulmonary disease have a poorer prognosis if pulmonary toxicity develops.
Hypersensitivity pneumonitis usually appears earlier in the course of therapy, and rechallenging these patients with amiodarone hydrochloride results in a more rapid recurrence of greater severity.
Bronchoalveolar lavage is the procedure of choice to confirm this diagnosis, which can be made when a T suppressor/cytotoxic (CD8-positive) lymphocytosis is noted. Steroid therapy should be instituted and amiodarone hydrochloride therapy discontinued in these patients.
Interstitial/alveolar pneumonitis may result from the release of oxygen radicals and/or phospholipidosis and is characterized by findings of diffuse alveolar damage, interstitial pneumonitis or fibrosis in lung biopsy specimens. Phospholipidosis (foamy cells, foamy macrophages), due to inhibition of phospholipase, will be present in most cases of amiodarone hydrochloride-induced pulmonary toxicity; however, these changes also are present in approximately 50% of all patients on amiodarone hydrochloride therapy. These cells should be used as markers of therapy, but not as evidence of toxicity. A diagnosis of amiodarone hydrochloride-induced interstitial/alveolar pneumonitis should lead, at a minimum, to dose reduction or, preferably, to withdrawal of the amiodarone hydrochloride to establish reversibility, especially if other acceptable antiarrhythmic therapies are available. Where these measures have been instituted, a reduction in symptoms of amiodarone-induced pulmonary toxicity was usually noted within the first week, and a clinical improvement was greatest in the first two to three weeks. Chest X-ray changes usually resolve within two to four months. According to some experts, steroids may prove beneficial. Prednisone in doses of 40 to 60 mg/day or equivalent doses of other steroids have been given and tapered over the course of several weeks depending upon the condition of the patient. In some cases rechallenge with amiodarone hydrochloride at a lower dose has not resulted in return of toxicity.
In a patient receiving amiodarone hydrochloride, any new respiratory symptoms should suggest the possibility of pulmonary toxicity, and the history, physical exam, chest X-ray, and pulmonary-function tests (with diffusion capacity) should be repeated and evaluated. A 15% decrease in diffusion capacity has a high sensitivity but only a moderate specificity for pulmonary toxicity; as the decrease in diffusion capacity approaches 30%, the sensitivity decreases but the specificity increases. A gallium-scan also may be performed as part of the diagnostic workup.
Fatalities, secondary to pulmonary toxicity, have occurred in approximately 10% of cases. However, in patients with life-threatening arrhythmias, discontinuation of amiodarone hydrochloride therapy due to suspected drug-induced pulmonary toxicity should be undertaken with caution, as the most common cause of death in these patients is sudden cardiac death. Therefore, every effort should be made to rule out other causes of respiratory impairment (i.e., congestive heart failure with Swan-Ganz catheterization if necessary, respiratory infection, pulmonary embolism, malignancy, etc.) before discontinuing amiodarone hydrochloride in these patients. In addition, bronchoalveolar lavage, transbronchial lung biopsy and/or open lung biopsy may be necessary to confirm the diagnosis, especially in those cases where no acceptable alternative therapy is available.
If a diagnosis of amiodarone hydrochloride-induced hypersensitivity pneumonitis is made, amiodarone hydrochloride should be discontinued, and treatment with steroids should be instituted. If a diagnosis of amiodarone hydrochloride-induced interstitial/alveolar pneumonitis is made, steroid therapy should be instituted and, preferably, amiodarone hydrochloride discontinued or, at a minimum, reduced in dosage. Some cases of amiodarone hydrochloride-induced interstitial/alveolar pneumonitis may resolve following a reduction in amiodarone hydrochloride dosage in conjunction with the administration of steroids. In some patients, rechallenge at a lower dose has not resulted in return of interstitial/alveolar pneumonitis; however, in some patients (perhaps because of severe alveolar damage) the pulmonary lesions have not been reversible.Worsened Arrhythmia
Amiodarone hydrochloride, like other antiarrhythmics, can cause serious exacerbation of the presenting arrhythmia and has been reported in about 2 to 5% in most series, and has included new ventricular fibrillation, incessant ventricular tachycardia, increased resistance to cardioversion, and polymorphic ventricular tachycardia associated with QTc prolongation (Torsade de Pointes [TdP]). In addition, amiodarone hydrochloride has caused symptomatic bradycardia or sinus arrest with suppression of escape foci in 2 to 4% of patients. The risk of exacerbation may be increased when other risk factors are present such as electrolytic disorders or use of concomitant antiarrhythmics or other interacting drugs (See “Drug Interactions”).
Correct hypokalemia, hypomagnesemia or hypocalcemia whenever possible before initiating treatment with amiodarone hydrochloride, as these disorders can exaggerate the degree of QTc prolongation and increase the potential for TdP. Give special attention to electrolyte and acid-base balance in patients experiencing severe or prolonged diarrhea or in patients receiving concomitant diuretics and laxatives, systemic corticosteroids, amphotericin B (IV) or other drugs affecting electrolyte levels.
The need to co-administer amiodarone with any other drug known to prolong the QTc interval must be based on a careful assessment of the potential risks and benefits of doing so for each patient.
Serious Symptomatic Bradycardia When Co-administered with Ledipasvir/Sofosbuvir or with Sofosbuvir with Simeprevir
Postmarketing cases of symptomatic bradycardia, some requiring pacemaker insertion and at least one fatal, have been reported when ledipasvir/sofosbuvir or sofosbuvir with simeprevir were initiated in patients on amiodarone. Bradycardia generally occurred within hours to days, but in some cases up to 2 weeks after initiating antiviral treatment. Bradycardia generally resolved after discontinuation of antiviral treatment. The mechanism for this effect is unknown. Monitor heart rate in patients taking or recently discontinuing amiodarone when starting antiviral treatment.
Implantable Cardiac Devices
In patients with implanted defibrillators or pacemakers, chronic administration of antiarrhythmic drugs may affect pacing or defibrillating thresholds. Therefore, at the inception of and during amiodarone treatment, pacing and defibrillation thresholds should be assessed.
Thyrotoxicosis
Amiodarone hydrochloride-induced hyperthyroidism may result in thyrotoxicosis and/or the possibility of arrhythmia breakthrough or aggravation. There have been reports of death associated with amiodarone-induced thyrotoxicosis. IF ANY NEW SIGNS OF ARRHYTHMIA APPEAR, THE POSSIBILITY OF HYPERTHYROIDISM SHOULD BE CONSIDERED (see “PRECAUTIONS, Thyroid Abnormalities”).
Liver Injury
Elevations of hepatic enzyme levels are seen frequently in patients exposed to amiodarone hydrochloride and in most cases are asymptomatic. If the increase exceeds three times normal, or doubles in a patient with an elevated baseline, discontinuation of amiodarone hydrochloride or dosage reduction should be considered. In a few cases in which biopsy has been done, the histology has resembled that of alcoholic hepatitis or cirrhosis. Hepatic failure has been a rare cause of death in patients treated with amiodarone hydrochloride.
Loss of Vision
Cases of optic neuropathy and/or optic neuritis, usually resulting in visual impairment, have been reported in patients treated with amiodarone. In some cases, visual impairment has progressed to permanent blindness. Optic neuropathy and/or neuritis may occur at any time following initiation of therapy. A causal relationship to the drug has not been clearly established. If symptoms of visual impairment appear, such as changes in visual acuity and decreases in peripheral vision, prompt ophthalmic examination is recommended. Appearance of optic neuropathy and/or neuritis calls for re-evaluation of amiodarone hydrochloride therapy. The risks and complications of antiarrhythmic therapy with amiodarone hydrochloride must be weighed against its benefits in patients whose lives are threatened by cardiac arrhythmias. Regular ophthalmic examination, including funduscopy and slit-lamp examination, is recommended during administration of amiodarone hydrochloride. (See “ADVERSE REACTIONS”).
Neonatal Injury
Amiodarone can cause fetal harm when administered to a pregnant woman. Fetal exposure may increase the potential for adverse experiences including cardiac, thyroid, neurodevelopmental, neurological and growth effects in neonate. Inform the patient of the potential hazard to the fetus if amiodarone hydrochloride is administered during pregnancy or if the patient becomes pregnant while taking amiodarone hydrochloride.
In pregnant rats and rabbits, amiodarone hydrochloride in doses of 25 mg/kg/day (approximately 0.4 and 0.9 times, respectively, the maximum recommended human maintenance dose*) had no adverse effects on the fetus. In the rabbit, 75 mg/kg/day (approximately 2.7 times the maximum recommended human maintenance dose*) caused abortions in greater than 90% of the animals. In the rat, doses of 50 mg/kg/day or more were associated with slight displacement of the testes and an increased incidence of incomplete ossification of some skull and digital bones; at 100 mg/kg/day or more, fetal body weights were reduced; at 200 mg/kg/day, there was an increased incidence of fetal resorption. (These doses in the rat are approximately 0.8, 1.6 and 3.2 times the maximum recommended human maintenance dose.*) Adverse effects on fetal growth and survival also were noted in one of two strains of mice at a dose of 5 mg/kg/day (approximately 0.04 times the maximum recommended human maintenance dose*).
*600 mg in a 50 kg patient (doses compared on a body surface area basis) -
PRECAUTIONS
Impairment of Vision
Optic Neuropathy and/or Neuritis
Cases of optic neuropathy and optic neuritis have been reported (see “WARNINGS”).
Corneal Microdeposits
Corneal microdeposits appear in the majority of adults treated with amiodarone hydrochloride. They are usually discernible only by slit-lamp examination, but give rise to symptoms such as visual halos or blurred vision in as many as 10% of patients. Corneal microdeposits are reversible upon reduction of dose or termination of treatment. Asymptomatic microdeposits alone are not a reason to reduce dose or discontinue treatment (see “ADVERSE REACTIONS”).
Neurologic
Chronic administration of oral amiodarone in rare instances may lead to the development of peripheral neuropathy that may resolve when amiodarone is discontinued, but this resolution has been slow and incomplete.
Photosensitivity
Amiodarone hydrochloride has induced photosensitization in about 10% of patients; some protection may be afforded by the use of sun-barrier creams or protective clothing. During long-term treatment, a blue-gray discoloration of the exposed skin may occur. The risk may be increased in patients of fair complexion or those with excessive sun exposure, and may be related to cumulative dose and duration of therapy.Thyroid Abnormalities
Amiodarone hydrochloride inhibits peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and may cause increased thyroxine levels, decreased T3 levels, and increased levels of inactive reverse T3 (rT3) in clinically euthyroid patients. It is also a potential source of large amounts of inorganic iodine. Because of its release of inorganic iodine, or perhaps for other reasons, amiodarone hydrochloride can cause either hypothyroidism or hyperthyroidism. Thyroid function should be monitored prior to treatment and periodically thereafter, particularly in elderly patients, and in any patient with a history of thyroid nodules, goiter, or other thyroid dysfunction. Because of the slow elimination of amiodarone hydrochloride and its metabolites, high plasma iodide levels, altered thyroid function, and abnormal thyroid-function tests may persist for several weeks or even months following amiodarone hydrochloride withdrawal.
Hypothyroidism has been reported in 2 to 10% of patients receiving amiodarone and may be primary or subsequent to resolution of preceding amiodarone-induced hyperthyroidism. This condition may be identified by clinical symptoms and elevated serum TSH levels. Cases of severe hypothyroidism and myxedema coma, sometimes fatal, have been reported in association with amiodarone therapy. In some clinically hypothyroid amiodarone-treated patients, free thyroxine index values may be normal. Manage hypothyroidism by reducing the dose of or discontinuing amiodarone hydrochloride and considering the need for thyroid hormone supplement.
Hyperthyroidism occurs in about 2% of patients receiving amiodarone hydrochloride, but the incidence may be higher among patients with prior inadequate dietary iodine intake. Amiodarone hydrochloride-induced hyperthyroidism usually poses a greater hazard to the patient than hypothyroidism because of the possibility of thyrotoxicosis and/or arrhythmia breakthrough or aggravation, all of which may result in death. There have been reports of death associated with amiodarone-induced thyrotoxicosis. IF ANY NEW SIGNS OF ARRHYTHMIA APPEAR, THE POSSIBILITY OF HYPERTHYROIDISM SHOULD BE CONSIDERED.
Hyperthyroidism is best identified by relevant clinical symptoms and signs, accompanied usually by abnormally elevated levels of serum T3 RIA, and further elevations of serum T4, and a subnormal serum TSH level (using a sufficiently sensitive TSH assay). The finding of a flat TSH response to TRH is confirmatory of hyperthyroidism and may be sought in equivocal cases. Since arrhythmia breakthroughs may accompany amiodarone hydrochloride-induced hyperthyroidism, aggressive medical treatment is indicated, including, if possible, dose reduction or withdrawal of amiodarone hydrochloride.
The institution of antithyroid drugs, β-adrenergic blockers and/or temporary corticosteroid therapy may be necessary. The action of antithyroid drugs may be especially delayed in amiodarone-induced thyrotoxicosis because of substantial quantities of preformed thyroid hormones stored in the gland. Radioactive iodine therapy is contraindicated because of the low radioiodine uptake associated with amiodarone-induced hyperthyroidism. Amiodarone hydrochloride-induced hyperthyroidism may be followed by a transient period of hypothyroidism (see “WARNINGS, Thyrotoxicosis”).
When aggressive treatment of amiodarone-induced thyrotoxicosis has failed or amiodarone cannot be discontinued because it is the only drug effective against the resistant arrhythmia, surgical management may be an option. Experience with thyroidectomy as a treatment for amiodarone-induced thyrotoxicosis is limited, and this form of therapy could induce thyroid storm. Therefore, surgical and anesthetic management require careful planning.
There have been postmarketing reports of thyroid nodules/thyroid cancer in patients treated with amiodarone hydrochloride. In some instances hyperthyroidism was also present (see “WARNINGS” and “ADVERSE REACTIONS”).Surgery
Volatile Anesthetic Agents: Close perioperative monitoring is recommended in patients undergoing general anesthesia who are on amiodarone therapy as they may be more sensitive to the myocardial depressant and conduction effects of halogenated inhalational anesthetics.
Hypotension Postbypass: Rare occurrences of hypotension upon discontinuation of cardiopulmonary bypass during open-heart surgery in patients receiving amiodarone hydrochloride have been reported. The relationship of this event to amiodarone hydrochloride therapy is unknown.
Adult Respiratory Distress Syndrome (ARDS): Postoperatively, occurrences of ARDS have been reported in patients receiving amiodarone hydrochloride therapy who have undergone either cardiac or noncardiac surgery. Although patients usually respond well to vigorous respiratory therapy, in rare instances the outcome has been fatal. Until further studies have been performed, it is recommended that FiO2 and the determinants of oxygen delivery to the tissues (e.g., SaO2, PaO2) be closely monitored in patients on amiodarone hydrochloride.
Corneal Refractive Laser Surgery
Patients should be advised that most manufacturers of corneal refractive laser surgery devices contraindicate that procedure in patients taking amiodarone hydrochloride.Information for Patients
Patients should be instructed to read the accompanying Medication Guide each time they refill their prescription. The complete text of the Medication Guide is reprinted at the end of this document.
Laboratory Tests
Elevations in liver enzymes (aspartate aminotransferase and alanine aminotransferase) can occur. Liver enzymes in patients on relatively high maintenance doses should be monitored on a regular basis. Persistent significant elevations in the liver enzymes or hepatomegaly should alert the physician to consider reducing the maintenance dose of amiodarone hydrochloride or discontinuing therapy.
Amiodarone hydrochloride alters the results of thyroid-function tests, causing an increase in serum T4 and serum reverse T3, and a decline in serum T3 levels. Despite these biochemical changes, most patients remain clinically euthyroid.Drug Interactions
In view of the long and variable half-life of amiodarone, potential for drug interactions exists, not only with concomitant medication, but also with drugs administered after discontinuation of amiodarone.
Pharmacodynamic interactions
Drugs inducing TdP or prolonging QT
Co-administration of amiodarone with drugs known to prolong the QT interval (such as class I and III antiarrhythmics, lithium, certain phenothiazines, tricyclic antidepressants, certain fluoroquinolone and macrolide antibiotics, IV pentamidine, and azole antifungals) increases the risk of Torsades de Points. Avoid concomitant use of drugs that prolong the QT interval.
Drugs lowering heart rate or causing automaticity or conduction disorders
Concomitant use of drugs with depressant effects on the sinus and AV node (e.g., digoxin, beta blockers, verapamil, diltiazem, clonidine) can potentiate the electrophysiologic and hemodynamic effects of amiodarone, resulting in bradycardia, sinus arrest, and AV block. Monitor heart rate in patients on amiodarone and concomitant drugs that slow heart rate.
Pharmacokinetic interactions
Effects of other medicinal products on amiodarone
Since amiodarone is a substrate for CYP3A and CYP2C8, drugs/substances that inhibit CYP3A (e.g., certain protease inhibitors, loratadine, cimetidine, trazodone) may decrease the metabolism and increase serum concentrations of amiodarone. Concomitant use of CYP3A inducers (rifampin, St. John’s Wort), may lead to decreased serum concentrations and loss of efficacy. Consider serial measurement of amiodarone serum concentration during concomitant use of drugs affecting CYP3A activity.
Grapefruit juice given to healthy volunteers increased amiodarone AUC by 50% and Cmax by 84%, and decreased DEA to unquantifiable concentrations. Grapefruit juice inhibits CYP3A-mediated metabolism of oral amiodarone in the intestinal mucosa, resulting in increased plasma levels of amiodarone; therefore, grapefruit juice should not be taken during treatment with oral amiodarone. This information should be considered when transitioning from intravenous to oral amiodarone. Cholestyramine reduces enterohepatic circulation of amiodarone thereby increasing its elimination. This results in reduced amiodarone serum levels and half-life.
Effects of amiodarone on other medicinal products
Amiodarone inhibits P-glycoprotein and certain CYP450 enzymes, including CYP1A2, CYP2C9, CYP2D6, and CYP3A. This inhibition can result in unexpectedly high plasma levels of other drugs which are metabolized by those CYP450 enzymes or are substrates of P-glycoprotein. Reported examples of this interaction include the following:
Cyclosporine (CYP3A substrate) administered in combination with oral amiodarone has been reported to produce persistently elevated plasma concentrations of cyclosporine resulting in elevated creatinine, despite reduction in dose of cyclosporine. Monitor cyclosporine drug levels and renal function in patients taking both drugs.
HMG-CoA reductase inhibitors: The use of HMG-CoA reductase inhibitors that are CYP3A substrates in combination with amiodarone has been associated with reports of myopathy/rhabdomyolysis. Limit the dose of simvastatin in patients on amiodarone to 20 mg daily. Limit the daily dose of lovastatin to 40 mg. Lower starting and maintenance doses of other CYP3A substrates (e.g., atorvastatin) may be required as amiodarone may increase the plasma concentration of these drugs.
Digoxin: In patients receiving digoxin therapy, administration of oral amiodarone results in an increase in the serum digoxin concentration. Amiodarone taken concomitantly with digoxin increases the serum digoxin concentration by 70% after one day. On initiation of oral amiodarone, the need for digitalis therapy should be reviewed and the dose reduced by approximately 50% or discontinued. If digitalis treatment is continued, serum levels should be closely monitored and patients observed for clinical evidence of toxicity.
Antiarrhythmics: The metabolism of quinidine, procainamide, flecainide can be inhibited by amiodarone. Amiodarone taken concomitantly with quinidine increases quinidine serum concentration by 33% after two days. Amiodarone taken concomitantly with procainamide for less than seven days increases plasma concentrations of procainamide and n-acetyl procainamide by 55% and 33%, respectively. In general, any added antiarrhythmic drug should be initiated at a lower than usual dose with careful monitoring.
Combination of amiodarone with other antiarrhythmic therapy should be reserved for patients with life-threatening ventricular arrhythmias who are incompletely responsive to a single agent or incompletely responsive to amiodarone. During transition to amiodarone the dose levels of previously administered agents should be reduced by 30 to 50% several days after the addition of amiodarone, when arrhythmia suppression should be beginning. The continued need for the other antiarrhythmic agent should be reviewed after the effects of amiodarone have been established, and discontinuation ordinarily should be attempted. If the treatment is continued, these patients should be particularly carefully monitored for adverse effects, especially conduction disturbances and exacerbation of tachyarrhythmias, as amiodarone is continued. In amiodarone-treated patients who require additional antiarrhythmic therapy, the initial dose of such agents should be approximately half of the usual recommended dose.
Metabolism of lidocaine (CYP3A substrate) can be inhibited by amiodarone resulting in increased lidocaine concentrations. Sinus bradycardia and seizure has been reported in patients receiving concomitant lidocaine and amiodarone.
Anticoagulants: Potentiation of warfarin-type (CYP2C9 and CYP3A substrate) anticoagulant response is almost always seen in patients receiving amiodarone and can result in serious or fatal bleeding. Since the concomitant administration of warfarin with amiodarone increases the prothrombin time by 100% after 3 to 4 days, the dose of the anticoagulant should be reduced by one-third to one-half, and prothrombin times should be monitored closely.
A potential interaction between clopidogrel and amiodarone resulting in ineffective inhibition of platelet aggregation has been reported.
Dabigatran etexilate when taken concomitantly with amiodarone may result in elevated serum concentration of dabigatran.
Fentanyl (CYP3A substrate) in combination with amiodarone may cause hypotension, bradycardia, and decreased cardiac output.
Increased steady-state levels of phenytoin during concomitant therapy with amiodarone have been reported. Monitor phenytoin levels in patients taking both drugs.
Dextromethorphan is a substrate for both CYP2D6 and CYP3A. Amiodarone inhibits CYP2D6 and CYP3A. Chronic (>2 weeks) amiodarone treatment impairs metabolism of dextromethorphan leading to increased serum concentration.Carcinogenesis, Mutagenesis, Impairment of Fertility
Amiodarone hydrochloride was associated with a statistically significant, dose-related increase in the incidence of thyroid tumors (follicular adenoma and/or carcinoma) in rats. The incidence of thyroid tumors was greater than control even at the lowest dose level tested, i.e., 5 mg/kg/day (approximately 0.08 times the maximum recommended human maintenance dose*).
Mutagenicity studies (Ames, micronucleus, and lysogenic tests) with amiodarone hydrochloride were negative.
In a study in which amiodarone hydrochloride was administered to male and female rats, beginning 9 weeks prior to mating, reduced fertility was observed at a dose level of 90 mg/kg/day (approximately 1.4 times the maximum recommended human maintenance dose*).
*600 mg in a 50 kg patient (dose compared on a body surface area basis)Pregnancy:
See “WARNINGS, Neonatal Injury”.
Teratogenic Effects
Amiodarone and desethylamiodarone cross the placenta.
Reported risks include:
- neonatal bradycardia, QT prolongation, and periodic ventricular extrasystoles
- neonatal hypothyroidism (with or without goiter) detected antenatally or in the newborn and reported even after a few days of exposure
- neonatal hyperthyroxinemia
- neurodevelopmental abnormalities independent of thyroid function, including speech delay and difficulties with written language and arithmetic, delayed motor development, and ataxia.
- jerk nystagmus with synchronous head titubation
- fetal growth retardation
- premature birth
Labor and Delivery
It is not known whether the use of amiodarone hydrochloride during labor or delivery has any immediate or delayed adverse effects. Preclinical studies in rodents have not shown any effect of amiodarone hydrochloride on the duration of gestation or on parturition.
Nursing Mothers
Amiodarone and one of its major metabolites, DEA, are excreted in human milk, suggesting that breast-feeding could expose the nursing infant to a significant dose of the drug. Nursing offspring of lactating rats administered amiodarone have been shown to be less viable and have reduced body-weight gains. The risk of exposing the infant to amiodarone and DEA must be weighed against the potential benefit of arrhythmia suppression in the mother. Advise the mother to discontinue nursing.
Pediatric Use
The safety and effectiveness of amiodarone hydrochloride tablets in pediatric patients have not been established.
Geriatric Use
Clinical studies of amiodarone hydrochloride tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Adverse reactions have been very common in virtually all series of patients treated with amiodarone hydrochloride for ventricular arrhythmias with relatively large doses of drug (400 mg/day and above), occurring in about three-fourths of all patients and causing discontinuation in 7 to 18%. The most serious reactions are pulmonary toxicity, exacerbation of arrhythmia, and rare serious liver injury (see “WARNINGS”), but other adverse effects constitute important problems. They are often reversible with dose reduction or cessation of amiodarone hydrochloride treatment. Most of the adverse effects appear to become more frequent with continued treatment beyond six months, although rates appear to remain relatively constant beyond one year. The time and dose relationships of adverse effects are under continued study.
Neurologic problems are extremely common, occurring in 20 to 40% of patients and including malaise and fatigue, tremor and involuntary movements, poor coordination and gait, and peripheral neuropathy; they are rarely a reason to stop therapy and may respond to dose reductions or discontinuation (see “PRECAUTIONS”). There have been spontaneous reports of demyelinating polyneuropathy.
Gastrointestinal complaints, most commonly nausea, vomiting, constipation, and anorexia, occur in about 25% of patients but rarely require discontinuation of drug. These commonly occur during high-dose administration (i.e., loading dose) and usually respond to dose reduction or divided doses.
Ophthalmic abnormalities including optic neuropathy and/or optic neuritis, in some cases progressing to permanent blindness, papilledema, corneal degeneration, photosensitivity, eye discomfort, scotoma, lens opacities, and macular degeneration have been reported. (See “WARNINGS”.)
Asymptomatic corneal microdeposits are present in virtually all adult patients who have been on drug for more than 6 months. Some patients develop eye symptoms of halos, photophobia, and dry eyes. Vision is rarely affected and drug discontinuation is rarely needed.
Dermatological adverse reactions occur in about 15% of patients, with photosensitivity being most common (about 10%). Sunscreen and protection from sun exposure may be helpful, and drug discontinuation is not usually necessary. Prolonged exposure to amiodarone hydrochloride occasionally results in a blue-gray pigmentation. This is slowly and occasionally incompletely reversible on discontinuation of drug but is of cosmetic importance only.
Cardiovascular adverse reactions, other than exacerbation of the arrhythmias, include the uncommon occurrence of congestive heart failure (3%) and bradycardia. Bradycardia usually responds to dosage reduction but may require a pacemaker for control. CHF rarely requires drug discontinuation. Cardiac conduction abnormalities occur infrequently and are reversible on discontinuation of drug.
The following side-effect rates are based on a retrospective study of 241 patients treated for 2 to 1,515 days (mean 441.3 days).
The following side effects were each reported in 10 to 33% of patients:
Gastrointestinal: Nausea and vomiting.
The following side effects were each reported in 4 to 9% of patients:
Dermatologic: Solar dermatitis/photosensitivity.
Neurologic: Malaise and fatigue, tremor/abnormal involuntary movements, lack of coordination, abnormal gait/ataxia, dizziness, paresthesias.
Gastrointestinal: Constipation, anorexia.
Ophthalmologic: Visual disturbances.
Hepatic: Abnormal liver-function tests.
Respiratory: Pulmonary inflammation or fibrosis.
The following side effects were each reported in 1 to 3% of patients:
Thyroid: Hypothyroidism, hyperthyroidism.
Neurologic: Decreased libido, insomnia, headache, sleep disturbances.
Cardiovascular: Congestive heart failure, cardiac arrhythmias, SA node dysfunction.
Gastrointestinal: Abdominal pain.
Hepatic: Nonspecific hepatic disorders.
Other: Flushing, abnormal taste and smell, edema, abnormal salivation, coagulation abnormalities.
The following side effects were each reported in less than 1% of patients:
Blue skin discoloration, rash, spontaneous ecchymosis, alopecia, hypotension, and cardiac conduction abnormalities.
In surveys of almost 5,000 patients treated in open U.S. studies and in published reports of treatment with amiodarone hydrochloride, the adverse reactions most frequently requiring discontinuation of amiodarone hydrochloride included pulmonary infiltrates or fibrosis, paroxysmal ventricular tachycardia, congestive heart failure, and elevation of liver enzymes. Other symptoms causing discontinuations less often included visual disturbances, solar dermatitis, blue skin discoloration, hyperthyroidism, and hypothyroidism.Postmarketing Reports
In postmarketing surveillance, serious symptomatic bradycardia has been reported in patients taking amiodarone who initiate treatment with ledipasvir/sofosbuvir or with sofosbuvir with simeprevir, hypotension (sometimes fatal), sinus arrest, anaphylactic/anaphylactoid reaction (including shock), angioedema, urticaria, eosinophilic pneumonia, hepatitis, cholestatic hepatitis, cirrhosis, pancreatitis, acute pancreatitis, renal impairment, renal insufficiency, acute renal failure, acute respiratory distress syndrome in the post-operative setting, bronchospasm, possibly fatal respiratory disorders (including distress, failure, arrest, and ARDS), bronchiolitis obliterans organizing pneumonia (possibly fatal), fever, dyspnea, cough, hemoptysis, wheezing, hypoxia, pulmonary infiltrates and/or mass, pulmonary alveolar hemorrhage, pleural effusion, pleuritis, pseudotumor cerebri, parkinsonian symptoms such as akinesia and bradykinesia (sometimes reversible with discontinuation of therapy), syndrome of inappropriate antidiuretic hormone secretion (SIADH), thyroid nodules/thyroid cancer, toxic epidermal necrolysis (sometimes fatal), erythema multiforme, Stevens-Johnson syndrome, exfoliative dermatitis, bullous dermatitis, drug rash with eosinophilia and systemic symptoms (DRESS), eczema, skin cancer, vasculitis, pruritus, hemolytic anemia, aplastic anemia, pancytopenia, neutropenia, thrombocytopenia, agranulocytosis, granuloma, myopathy, muscle weakness, rhabdomyolysis, demyelinating polyneuropathy, hallucination, confusional state, disorientation, delirium, epididymitis, impotence, dry mouth, and lupus-like syndrome, also have been reported with amiodarone therapy.
-
OVERDOSAGE
There have been cases, some fatal, of amiodarone hydrochloride overdose.
In addition to general supportive measures, the patient’s cardiac rhythm and blood pressure should be monitored, and if bradycardia ensues, a β-adrenergic agonist or a pacemaker may be used. Hypotension with inadequate tissue perfusion should be treated with positive inotropic and/or vasopressor agents. Neither amiodarone hydrochloride nor its metabolite is dialyzable.
The acute oral LD50 of amiodarone hydrochloride in mice and rats is greater than 3,000 mg/kg. -
DOSAGE AND ADMINISTRATION
BECAUSE OF THE UNIQUE PHARMACOKINETIC PROPERTIES, DIFFICULT DOSING SCHEDULE, AND SEVERITY OF THE SIDE EFFECTS IF PATIENTS ARE IMPROPERLY MONITORED, AMIODARONE HYDROCHLORIDE SHOULD BE ADMINISTERED ONLY BY PHYSICIANS WHO ARE EXPERIENCED IN THE TREATMENT OF LIFE-THREATENING ARRHYTHMIAS WHO ARE THOROUGHLY FAMILIAR WITH THE RISKS AND BENEFITS OF AMIODARONE HYDROCHLORIDE THERAPY, AND WHO HAVE ACCESS TO LABORATORY FACILITIES CAPABLE OF ADEQUATELY MONITORING THE EFFECTIVENESS AND SIDE EFFECTS OF TREATMENT.
In order to insure that an antiarrhythmic effect will be observed without waiting several months, loading doses are required. A uniform, optimal dosage schedule for administration of amiodarone hydrochloride has not been determined. Because of the food effect on absorption, amiodarone hydrochloride should be administered consistently with regard to meals (see “CLINICAL PHARMACOLOGY”). Individual patient titration is suggested according to the following guidelines:
For life-threatening ventricular arrhythmias, such as ventricular fibrillation or hemodynamically unstable ventricular tachycardia: Close monitoring of the patients is indicated during the loading phase, particularly until risk of recurrent ventricular tachycardia or fibrillation has abated. Because of the serious nature of the arrhythmia and the lack of predictable time course of effect, loading should be performed in a hospital setting. Loading doses of 800 to 1,600 mg/day are required for 1 to 3 weeks (occasionally longer) until initial therapeutic response occurs. (Administration of amiodarone hydrochloride in divided doses with meals is suggested for total daily doses of 1,000 mg or higher, or when gastrointestinal intolerance occurs.) If side effects become excessive, the dose should be reduced. Elimination of recurrence of ventricular fibrillation and tachycardia usually occurs within 1 to 3 weeks, along with reduction in complex and total ventricular ectopic beats.
Since grapefruit juice is known to inhibit CYP3A4-mediated metabolism of oral amiodarone in the intestinal mucosa, resulting in increased plasma levels of amiodarone, grapefruit juice should not be taken during treatment with oral amiodarone (see “PRECAUTIONS, Drug Interactions”).
Upon starting amiodarone hydrochloride therapy, an attempt should be made to gradually discontinue prior antiarrhythmic drugs (see section on “Drug Interactions”). When adequate arrhythmia control is achieved, or if side effects become prominent, amiodarone hydrochloride dose should be reduced to 600 to 800 mg/day for one month and then to the maintenance dose, usually 400 mg/day (see “CLINICAL PHARMACOLOGY–Monitoring Effectiveness”). Some patients may require larger maintenance doses, up to 600 mg/day, and some can be controlled on lower doses. Amiodarone hydrochloride may be administered as a single daily dose, or in patients with severe gastrointestinal intolerance, as a b.i.d. dose. In each patient, the chronic maintenance dose should be determined according to antiarrhythmic effect as assessed by symptoms, Holter recordings, and/or programmed electrical stimulation and by patient tolerance. Plasma concentrations may be helpful in evaluating nonresponsiveness or unexpectedly severe toxicity (see “CLINICAL PHARMACOLOGY”).
The lowest effective dose should be used to prevent the occurrence of side effects. In all instances, the physician must be guided by the severity of the individual patient’s arrhythmia and response to therapy.
When dosage adjustments are necessary, the patient should be closely monitored for an extended period of time because of the long and variable half-life of amiodarone hydrochloride and the difficulty in predicting the time required to attain a new steady-state level of drug. Dosage suggestions are summarized below:
Loading Dose (Daily) Adjustment and Maintenance Dose (Daily) Ventricular Arrhythmias
1 to 3 weeks
~1 month
usual maintenance
800 to 1,600 mg
600 to 800 mg
400 mg
-
HOW SUPPLIED
Amiodarone Hydrochloride Tablets, 200 mg are white to off-white, round biconvex uncoated tablets debossed with “8” and “4” separated by a breakline on one side and “A” on the other side.
Blistercards of 30 NDC: 0615-8258-39
Blistercards of 15 NDC: 0615-8258-05
Keep tightly closed.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light.
Dispense in a light-resistant, tight container.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad - 500 038, India
Revised: 07/2018
Dispense with Medication Guide available at: www.aurobindousa.com/product-medication-guides -
Medication Guide
Amiodarone Hydrochloride Tablets
(A-mee-OH-da-rone HYE-droe-KLOR-ide)
What is the most important information I should know about amiodarone hydrochloride tablets?
Amiodarone hydrochloride tablets can cause serious side effects that can lead to death including:- lung problems
- liver problems
- worsening heartbeat problems
- thyroid problems
Call your doctor or get medical help right away if you have any of the following symptoms during treatment with amiodarone hydrochloride tablets:
- shortness of breath, wheezing, or any other trouble breathing; coughing, chest pain, or spitting up of blood
- nausea or vomiting, brown or dark-colored urine feel more tired than usual, yellowing of your skin or the whites of your eyes (jaundice), or right upper stomach pain
- heart pounding, skipping a beat, beating fast or slowly, feel light-headed or faint
- weakness, weight loss or weight gain, heat or cold intolerance, hair thinning, sweating, changes in your menses, swelling of your neck (goiter), nervousness, irritability, restlessness, decreased concentration, depression in the elderly, or tremor.
Amiodarone hydrochloride tablets should only be used in people with life-threatening heartbeat problems called ventricular arrhythmias, for which other treatments did not work or were not tolerated.
Amiodarone hydrochloride tablets can cause other serious side effects. See “What are the possible side effects of amiodarone hydrochloride tablets?” If you get serious side effects during treatment you may need to stop amiodarone hydrochloride tablets, have your dose changed, or get medical treatment. Talk with your doctor before you stop taking amiodarone hydrochloride tablets.
You may still have side effects after stopping amiodarone hydrochloride tablets because the medicine stays in your body months after treatment is stopped.
Tell all your healthcare providers that you take or took amiodarone hydrochloride tablets.
What are amiodarone hydrochloride tablets?
Amiodarone hydrochloride tablets are a prescription medicine used to treat life-threatening heartbeat problems called ventricular arrhythmias, for which other treatment did not work or was not tolerated. Amiodarone hydrochloride tablets have not been shown to help people with life-threatening heartbeat problems live longer. Amiodarone hydrochloride tablets should be started in a hospital to monitor your condition. You should have regular check-ups, blood tests, chest x-rays, and eye exams before and during treatment with amiodarone hydrochloride tablets to check for serious side effects.
It is not known if amiodarone hydrochloride tablets are safe and effective in children.
Who should not take amiodarone hydrochloride tablets?
Do not take amiodarone hydrochloride tablets if you:- have a certain heart condition called heart block with or without a slow heart rate
- have a slow heart rate with dizziness or lightheadedness, and you do not have an implanted pacemaker
- are allergic to amiodarone, iodine, or any of the other ingredients in amiodarone hydrochloride tablets. See the end of this Medication Guide for a complete list of ingredients in amiodarone hydrochloride tablets.
What should I tell my doctor before taking amiodarone hydrochloride tablets
Before you take amiodarone hydrochloride tablets tell your doctor about all of your medical conditions including if you:
- have lung or breathing problems
- have liver problems
- have or had thyroid problems
- have blood pressure problems
- are pregnant or plan to become pregnant. Amiodarone hydrochloride tablets can harm your unborn baby. Amiodarone hydrochloride can stay in your body for months after treatment is stopped. Talk with your doctor before you plan to get pregnant.
- are breastfeeding or plan to breastfeed. Amiodarone hydrochloride can pass into your breast milk and can harm your baby. Talk to your doctor about the best way to feed your baby. You should not breast feed while taking amiodarone hydrochloride tablets. Also, amiodarone hydrochloride can stay in your body for months after treatment is stopped.
- Tell your doctor about all the medicines you take including prescription and over-the-counter medicines, vitamins and herbal supplements. Amiodarone hydrochloride tablets and certain other medicines can affect (interact) with each other and cause serious side effects. You can ask your pharmacist for a list of medicines that interact with amiodarone hydrochloride tablets.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take amiodarone hydrochloride tablets?
- Take amiodarone hydrochloride tablets exactly as your doctor tells you to take them.
- Your doctor will tell you how much amiodarone hydrochloride to take and when to take it. Amiodarone hydrochloride tablets can be taken with or without food. Make sure you take amiodarone hydrochloride tablets the same way each time.
- If you take too much amiodarone hydrochloride, call your doctor or go to the nearest hospital emergency room right away.
- If you miss a dose, wait and take your next dose at your regular time. Do not take two doses at the same. Continue with your next regularly scheduled dose.
What should I avoid while taking amiodarone hydrochloride tablets?
- Do not drink grapefruit juice during treatment with amiodarone hydrochloride tablets. Grapefruit juice affects how amiodarone hydrochloride tablets are absorbed in the stomach.
Avoid sunlight. Amiodarone hydrochloride tablets can make your skin sensitive to sun and the light from sunlamps and tanning beds. You could get severe sunburn. Use sunscreen and wear a hat and clothes that cover your skin if you have to be in sunlight. Talk to you doctor if you get sunburn.
What are the possible side effects of amiodarone hydrochloride tablets?
See “What is the most important information I should know about amiodarone hydrochloride tablets?”
- vision problems that may lead to permanent blindness. You should have regular eye exams before and during treatment with amiodarone hydrochloride tablets. Call your doctor if you have blurred vision, see halos, or your eyes become sensitive to light. Tell your doctor if you plan to have laser eye surgery.
- nerve problems. Amiodarone hydrochloride tablets can cause a feeling of “pins and needles” or numbness in the hands, legs, or feet, muscle weakness, uncontrolled movements, poor coordination, and trouble walking.
- skin problems. Amiodarone hydrochloride tablets can cause your skin to be more sensitive to the sun or turn a bluish-gray color. In most people, skin color slowly returns to normal after stopping amiodarone hydrochloride tablets. In some people, skin color does not return to normal.
The most common side effects of amiodarone hydrochloride tablets include:
- nausea
- vomiting
- constipation
- loss of appetite.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of amiodarone hydrochloride tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store amiodarone hydrochloride tablets?- Store amiodarone hydrochloride tablets at room temperature between 20° to 25°C (68° to 77°F).
- Keep amiodarone hydrochloride tablets in a tightly closed container, and keep amiodarone hydrochloride tablets out of the light.
Keep amiodarone hydrochloride tablets and all medicines out of the reach of children.
General information about the safe and effective use of amiodarone hydrochloride tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use amiodarone hydrochloride tablets for a condition for which it was not prescribed. Do not give amiodarone hydrochloride tablets to other people, even if they have the same symptoms that you have. They may harm them.
If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about amiodarone hydrochloride tablets that was written for health professionals.
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
What are the ingredients in amiodarone hydrochloride tablets?
Active Ingredient: amiodarone hydrochloride
Inactive Ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, povidone, and sodium starch glycolate.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Dispense with Medication Guide available at: www.aurobindousa.com/product-medication-guides
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad - 500 038, India
Revised: 07/2018 - PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg
-
INGREDIENTS AND APPEARANCE
AMIODARONE HYDROCHLORIDE
amiodarone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0615-8258(NDC:65862-732) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIODARONE HYDROCHLORIDE (UNII: 976728SY6Z) (AMIODARONE - UNII:N3RQ532IUT) AMIODARONE HYDROCHLORIDE 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (White to Off-white) Score 2 pieces Shape ROUND (Biconvex) Size 11mm Flavor Imprint Code 8;4;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0615-8258-05 15 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/05/2019 2 NDC: 0615-8258-39 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/05/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204742 06/03/2016 Labeler - NCS HealthCare of KY, Inc dba Vangard Labs (050052943) Establishment Name Address ID/FEI Business Operations NCS HealthCare of KY, Inc dba Vangard Labs 050052943 REPACK(0615-8258)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.