ORALAIR- anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phelum pratense pollen, and poa pratensis pollen kit ORALAIR 300 IR- anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phleum pratense pollen, and poa pratensis pollen tablet, orally disintegrating
Oralair by
Drug Labeling and Warnings
Oralair by is a Other medication manufactured, distributed, or labeled by CENEXI HSC, CENEXI - Osny. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ORALAIR safely and effectively. See full prescribing information for ORALAIR.
ORALAIR ® (Sweet Vernal, Orchard, Perennial Rye, Timothy, and Kentucky Blue Grass Mixed Pollens Allergen Extract)
Tablet for Sublingual Use
Initial U.S. Approval: 2014WARNING: SEVERE ALLERGIC REACTIONS
See full prescribing information for complete boxed warning- ORALAIR can cause life-threatening allergic reactions such as anaphylaxis and severe laryngopharyngeal edema. (5.1)
- Do not administer ORALAIR to patients with severe, unstable or uncontrolled asthma. (4)
- Observe patients in the office for at least 30 minutes following the initial dose. (5.1)
- Prescribe auto-injectable epinephrine, instruct and train patients on its appropriate use, and instruct patients to seek immediate medical care upon its use. (5.2)
- ORALAIR may not be suitable for patients with certain underlying medical conditions that may reduce their ability to survive a serious allergic reaction. (5.2)
- ORALAIR may not be suitable for patients who may be unresponsive to epinephrine or inhaled bronchodilators, such as those taking beta-blockers. (5.2)
RECENT MAJOR CHANGES
Indications and Usage (1) 11/2018 Dosage and Administration (2.1) 11/2018 INDICATIONS AND USAGE
ORALAIR is an allergen extract indicated as immunotherapy for the treatment of grass pollen-induced allergic rhinitis with or without conjunctivitis confirmed by positive skin test or in vitro testing for pollen-specific IgE antibodies for any of the five grass species contained in this product. ORALAIR is approved for use in persons 5 through 65 years of age.
DOSAGE AND ADMINISTRATION
For sublingual use only.
Age
(years)Dose Day 1 Day 2 Day 3 and following 5-17 100 IR 2× 100 IR 300 IR 18-65 300 IR 300 IR 300 IR - Initiate treatment 4 months before the expected onset of each grass pollen season and continue treatment throughout the season. (2.2)
- Place the tablet under the tongue for at least 1 minute, until complete dissolution and then swallow. (2.2)
- Administer the first dose of ORALAIR under the supervision of a physician with experience in the diagnosis and treatment of severe allergic reactions. Observe the patient for at least 30 minutes. (2.1)
DOSAGE FORMS AND STRENGTHS
- Tablets, 100 IR and 300 IR (3)
CONTRAINDICATIONS
- Severe, unstable or uncontrolled asthma (4)
- History of any severe systemic allergic reaction or any severe local reaction to sublingual allergen immunotherapy (4)
- A history of eosinophilic esophagitis (4)
- Hypersensitivity to any of the inactive ingredients contained in this product (4)
WARNINGS AND PRECAUTIONS
- Inform patients of the signs and symptoms of severe allergic reactions and instruct them to seek immediate medical care and discontinue therapy should any of these occur. (5.1)
- In case of oral inflammation or wounds, stop treatment with ORALAIR to allow complete healing of the oral cavity. (5.5)
ADVERSE REACTIONS
Adverse reactions reported in > 5% of patients were: oral pruritus, throat irritation, ear pruritus, mouth edema, tongue pruritus, cough, asthma, oropharyngeal pain, tonsillitis, and oral paresthesia (6)
To report SUSPECTED ADVERSE REACTIONS, contact Stallergenes at 1-855-274-1322 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for Patient Counseling Information and FDA approved Medication Guide
-
TABLE OF CONTENTS
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SEVERE ALLERGIC REACTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Administration3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Allergic Reactions
5.2 Epinephrine
5.3 Eosinophilic Esophagitis
5.4 Asthma
5.5 Concomitant Allergen Immunotherapy
5.6 Oral Inflammation
5.7 Initiation of ORALAIR Therapy during Grass Pollen Season6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
* Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SEVERE ALLERGIC REACTIONS
- ORALAIR can cause life-threatening allergic reactions such as anaphylaxis and severe laryngopharyngeal restriction. (5.1)
- Do not administer ORALAIR to patients with severe, unstable or uncontrolled asthma. (4)
- Observe patients in the office for at least 30 minutes following the initial dose. (5.1)
- Prescribe auto-injectable epinephrine, instruct and train patients on its appropriate use, and instruct patients to seek immediate medical care upon its use. (5.2)
- ORALAIR may not be suitable for patients with certain underlying medical conditions that may reduce their ability to survive a serious allergic reaction. (5.2)
- ORALAIR may not be suitable for patients who may be unresponsive to epinephrine or inhaled bronchodilators, such as those taking beta-blockers. (5.2)
-
1 INDICATIONS AND USAGE
ORALAIR is an allergen extract indicated as immunotherapy for the treatment of grass pollen-induced allergic rhinitis with or without conjunctivitis confirmed by positive skin test or in vitro testing for pollen-specific IgE antibodies for any of the five grass species contained in this product. ORALAIR is approved for use in persons 5 through 65 years of age.
ORALAIR is not indicated for the immediate relief of allergy symptoms.
-
2 DOSAGE AND ADMINISTRATION
For sublingual use only.
2.1 Dose
For adults 18 through 65 years of age, the dose is 300 IR (index of reactivity) daily. For children and adolescents 5 through 17 years of age, the dose is increased over the first three days as shown in Table 1.
Table 1. Dosage for Adults and Children for the Days 1-3 (and following) Age (years) Dose Day 1 Day 2 Day 3 and following 5-17 100 IR 2x100 IR 300 IR 18-65 300 IR 300 IR 300 IR 2.2 Administration
Administer the first dose of ORALAIR in a healthcare setting in which acute allergic reactions can be treated under the supervision of a physician with experience in the diagnosis and treatment of severe allergic reactions. After receiving the first dose of ORALAIR, observe the patient for at least 30 minutes to monitor for signs or symptoms of a severe systemic or a severe local allergic reaction. If the patient tolerates the first dose, the patient may take subsequent doses at home.
Administer ORALAIR to children under adult supervision.
Remove the ORALAIR tablet from the blister just prior to dosing.
Place the ORALAIR tablet immediately under the tongue until complete dissolution for at least 1 minute before swallowing.
Wash hands after handling the ORALAIR tablet.
Do not take the ORALAIR tablet with food or beverage. To avoid swallowing allergen extract, food or beverage should not be taken for 5 minutes following dissolution of the tablet.
Initiate treatment 4 months before the expected onset of each grass pollen season and maintain it throughout the grass pollen season.
Data regarding the safety of starting treatment during the pollen season are not available. Data regarding the safety of restarting treatment after missing a dose of ORALAIR are not available.
It is recommended that auto-injectable epinephrine be made available to patients prescribed ORALAIR. Patients who are prescribed epinephrine while receiving immunotherapy should be instructed in the proper use of emergency self-injection of epinephrine [ See Warnings and Precautions (5.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ORALAIR is contraindicated in patients with:
- Severe, unstable or uncontrolled asthma
- History of any severe systemic allergic reaction
- History of any severe local reaction to sublingual allergen immunotherapy
- A history of eosinophilic esophagitis
- Hypersensitivity to any of the inactive ingredients (mannitol, microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous silica, magnesium stearate and lactose monohydrate) contained in this product [ See Description (11)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe Allergic Reactions
ORALAIR can cause systemic allergic reactions including anaphylaxis which may be life-threatening. In addition, ORALAIR can cause severe local reactions, including laryngopharyngeal swelling, which can compromise breathing and be life-threatening.
Patients who have a systemic allergic reaction to ORALAIR should stop taking ORALAIR.
Patients who have either escalating or persistent local reactions to ORALAIR should be reevaluated and considered for discontinuation of ORALAIR.
Administer the initial dose of ORALAIR in a healthcare setting under the supervision of a physician prepared to manage a severe
systemic or a severe local allergic reaction. Observe patients in the office for at least 30 minutes following the initial dose of ORALAIR.
Severe and serious allergic reactions may require treatment with epinephrine [ See Warnings and Precautions (5.2)].
5.2 Epinephrine
Prescribe auto-injectable epinephrine to patients receiving ORALAIR. Instruct patients to recognize the signs and symptoms of a severe allergic reaction and in the proper use of emergency self-injection of epinephrine, and instruct patients to seek immediate medical care upon its use [ See Patient Counseling Information (17)].
ORALAIR may not be suitable for patients with certain medical conditions that may reduce the ability to survive a serious allergic reaction or increase the risk of adverse reactions after epinephrine administration. Examples of these medical conditions include but are not limited to: markedly compromised lung function (either chronic or acute), unstable angina, recent myocardial infarction, significant arrhythmia, and uncontrolled hypertension.
ORALAIR may not be suitable for patients who are taking medications that can potentiate or inhibit the effect of epinephrine. These medications include:
Βeta-adrenergic blockers: Patients taking beta-adrenergic blockers may be unresponsive to the usual doses of epinephrine used to treat serious systemic reactions, including anaphylaxis. Specifically, beta-adrenergic blockers antagonize the cardiostimulating and bronchodilating effects of epinephrine.
Alpha-adrenergic blockers, ergot alkaloids: Patients taking alpha-adrenergic blockers may be unresponsive to the usual doses of epinephrine used to treat serious systemic reactions, including anaphylaxis. Specifically, alpha-adrenergic blockers antagonize the vasoconstricting and hypertensive effects of epinephrine. Similarly, ergot alkaloids may reverse the pressor effects of epinephrine.
Tricyclic antidepressants, levothyroxine sodium, monoamine oxidase inhibitors and certain antihistamines: The adverse effects of epinephrine may be potentiated in patients taking tricyclic antidepressants, levothyroxine sodium, monoamine oxidase inhibitors, and the antihistamines chlorpheniramine, and diphenhydramine.
Cardiac glycosides, diuretics: Patients who receive epinephrine while taking cardiac glycosides or diuretics should be observed carefully for the development of cardiac arrhythmias.
5.3 Eosinophilic Esophagitis
Eosinophilic esophagitis has been reported in association with sublingual tablet immunotherapy [ See Contraindications (4) and Adverse Reactions (6.2)]. Discontinue ORALAIR and consider a diagnosis of eosinophilic esophagitis in patients who experience severe or persistent gastro-esophageal symptoms including dysphagia or chest pain.
5.4 Asthma
ORALAIR has not been studied in subjects with moderate or severe asthma or any subjects who required daily medication.
Immunotherapy with ORALAIR should be withheld if the patient is experiencing an acute asthma exacerbation. Reevaluate patients who have recurrent asthma exacerbations and consider discontinuation of ORALAIR.
5.5 Concomitant Allergen Immunotherapy
ORALAIR has not been studied in subjects receiving concomitant allergen immunotherapy. Concomitant dosing with other allergen immunotherapy may increase the likelihood of local or systemic adverse reactions to either subcutaneous or sublingual allergen immunotherapy.
-
6 ADVERSE REACTIONS
Adverse reactions reported in > 5% of patients were: oral pruritus, throat irritation, ear pruritus, mouth edema, tongue pruritus, cough, oropharyngeal pain.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rate observed in practice.
Adults
Overall, in 6 placebo-controlled clinical trials, 1,038 adults 18 through 65 years of age received at least one dose of ORALAIR 300IR, of whom 611 (59%) completed at least four months of therapy. Of study participants, 56% were male, 17% had a history of mild intermittent asthma at study entry, and 64% were polysensitized. Data on race and ethnicity were not systematically captured in the five European studies (N=805). In the US study (N=233), a limited number of patients reported their race as other than White/Caucasian (Black/African American: 5.6%, Asian: 2.6%, Other: 2.1%) or their ethnicity as Hispanic or Latino (3.0%). Adverse events were captured on a daily diary card that did not solicit for specific adverse events.
Across the six clinical studies, adverse reactions reported at an incidence of > 2% of ORALAIR recipients and at a greater incidence than that in participants treated with placebo are listed in Table 2.
Table 2. Adverse Reactions Reported by ≥2% of Adults Receiving ORALAIR 300 IR and at a Greater Incidence than that in Participants Treated with Placebo Adverse Reactions ORALAIR 300 IR (N=1,038)
PLACEBO (N=840) Ear and labyrinth disorders Ear pruritus 8.4% 0.6% Respiratory, thoracic and mediastinal disorders Throat irritation 22.0% 3.7% Cough 7.3% 5.9% Oropharyngeal pain 5.1% 3.7% Pharyngeal edema 3.8% 0.1% Gastrointestinal disorders Oral pruritus 25.1% 5.0% Edema mouth 8.2% 0.6% Tongue pruritus 7.9% 0.7% Lip edema 4.4% 0.4% Paraesthesia oral 4.3% 1.0% Abdominal pain 4.2% 1.3% Dyspepsia 3.9% 0.4% Tongue edema 2.7% 0.1% Hypoaesthesia oral 2.2% 0.1% Stomatitis 2.1% 0.7% Skin and subcutaneous tissue disorders Urticaria 2.3% 1.5% Additional adverse reactions of interest that occurred in < 2% of ORALAIR recipients include dysphagia, nausea, vomiting, esophageal pain, gastritis, and gastroesophageal reflux.
Children and Adolescents
Overall, in placebo-controlled clinical trials, 154 children and adolescents 5 through 17 years of age received ORALAIR 300 IR, of whom 147 were exposed for more than 3 months. Of study participants, 66% were male, and 21% had a history of mild intermittent asthma at study entry. Data on race and ethnicity were not systematically captured.
The safety profile in the pediatric population, was generally similar to that of adults. In pediatric patients receiving ORALAIR, additional adverse reactions reported at an incidence of > 2% and at a greater incidence than that in participants treated with placebo are listed in Table 3.
Table 3. Additional Adverse Reactions Reported by ≥ 2% of Children and Adolescents Receiving ORALAIR 300 IR and at a Greater Incidence than that in Participants Treated with Placebo Adverse Rections ORALAIR 300 IR (N=154) PLACEBO (N=158) Infections and infestations Tonsillitis 5.8% 3.2% Upper respiratory tract infection 3.9% 1.9% Respiratory, thoracic and mediastinal disorders Asthma 7.1% 3.8% Dysphonia 2.6% 1.3% Gastrointestinal disorders Lip pruritus 3.2% 0.0% Skin and subcutaneous tissue disorders Atopic dermatitis 3.2% 0.6% An open-label study was conducted to evaluate the 30-day safety profile of ORLAIR in 307 children 5 through 9 years of age. Of study participants, 71% were male and 36% had a history of mild intermittent asthma at study entry. Data on race and ethnicity were not systematically captured. Adverse reactions reported at an incidence of > 2% were: throat irritation (22.1%), oral pruritus (11.7%), oral paresthesia (11.1%), tongue pruritus (8.1%), mouth edema (6.2%), cough (6.2%), oropharyngeal pain (4.2%), ear pruritus (5.2%), eye pruritus (4.6%), lip edema (3.3%), vomiting (2.6%), tongue edema (2.3%), abdominal pain (2.3%), oral discomfort (2.3%), and ocular hyperemia (2.0%).
Serious Adverse Reactions
At least 1 serious adverse event was reported in 22 of 1514 (1.5%) pediatric and adult subjects from randomized clinical trials who received ORALAIR at any dose, and 11 of 840 (1.1%) of placebo recipients. Of the 22 serious adverse events in the ORALAIR recipients, 2 were considered "definitely related" to ORALAIR.
The first subject was an adult who experienced a severe hypersensitivity reaction which began 5 minutes after administration of ORALAIR. The symptoms were violent coughing and marked dyspnea. The subject was treated with antihistamines, salbutamol and prednisolone and the reaction resolved without sequelae.The second subject was an adult who experienced severe laryngeal edema. The subject was treated with prednisolone and event resolved without sequelae.
There was also one case of gastroenteritis with an onset on Day 93 of therapy that was possibly related to ORALAIR.
In the open-label study conducted in 307 children 5 through 9 years of age, 2 serious adverse events were considered "likely" related to ORALAIR.
The first subject was an 8-year-old subject with a history of allergic bronchial asthma who developed oral pruritus, conjunctivitis, urticaria and asthma exacerbation 15 minutes after administration of ORALAIR on Day 5. The subject was treated with antihistamines and inhaled salbutamol and the reactions fully resolved in 30 minutes. The subject continued ORALAIR.
The second subject was a 6-year-old subject who developed severe lip, eye and eyelid swelling after administration of ORALAIR on Day 26 of therapy. The subject was treated with intravenous antihistamines and prednisolone. Reaction fully resolved within 6 hours. Treatment with ORALAIR was discontinued.
6.2 Postmarketing Experience
Post Marketing Safety Studies
A total of 1728 individuals (808 adults; 920 children 5 through 17 years of age) received ORALAIR in post marketing safety studies. Reported adverse reactions included: anaphylactic reaction, oral allergy syndrome, flushing, dyspnea, laryngeal edema, and diarrhea.
Spontaneous Postmarketing Reports
In addition to adverse reactions reported in clinical and post marketing safety studies, the following adverse reactions have been identified during post approval use of ORALAIR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: autoimmune thyroiditis, eosinophilic myocarditis, eosinophilic esophagitis, palpitations, tachycardia, hypotension, loss of consciousness, circulatory collapse, malaise, pallor, peripheral vascular disorder, stridor, angioedema, face edema, weight decreased, wheezing, exacerbation of asthma, chest discomfort, oropharyngeal paresthesia, oropharyngeal blistering, headache, dizziness, tinnitus, asthenia, somnolence, anxiety, rash, pruritus, salivary gland enlargement and/or hypersecretion, dry mouth, dry eye, influenza-like syndrome, lymphadenopathy, eosinophil count increased.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Available human data do not establish the presence or absence of ORALAIR-associated risks during pregnancy, labor and delivery.
Two developmental toxicity studies were performed in female rats and female rabbits given doses up to 250 IR/day and 3500 IR/day of ORALAIR, respectively, during gestation.Data
In developmental toxicity studies, the effect of ORALAIR on embryo/fetal development was evaluated in female rats and female rabbits administered with doses up to 250 IR/day and 3500 IR/day of ORALAIR, respectively, by oral gavage on days 6-17 of gestation for rats, and days 6-18 of gestation for rabbits. The human dose is 300 IR/day. There were no ORALAIR-related fetal malformations or other evidence of teratogenesis noted in these studies.8.2 Lactation
Risk Summary
Data are not available to assess the effects of ORALAIR on the breastfed child or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ORALAIR and any potential adverse effects on the breastfed child from ORALAIR or from the underlying maternal condition. -
11 DESCRIPTION
ORALAIR (Sweet Vernal, Orchard, Perennial Rye, Timothy, and Kentucky Blue Grass Mixed Pollens Allergen Extract) is a mixed allergen extract of the following five pollens: Sweet Vernal ( Anthoxanthum odoratum L), Orchard ( Dactylis glomerata L), Perennial Rye ( Lolium perenne L), Timothy ( Phleum pratense L), and Kentucky Blue Grass ( Poa pratensis L).
ORALAIR is available as a sublingual tablet in the following strengths:
- 100 IR (equivalent to approximately 3000 BAU (bioequivalent allergy units)
- 300 IR (equivalent to approximately 9000 BAU
Inactive ingredients: mannitol, microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous silica, magnesium stearate and lactose monohydrate.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies were conducted in animals. There was no evidence of mutagenic or clastogenic activity in response to ORALAIR in the in vitro bacterial mutagenesis assay and mouse lymphoma thymidine kinase cell assay or the in vivo bone marrow micronucleus and unscheduled DNA synthesis tests in rats.
No fertility study was conducted with ORALAIR.
-
14 CLINICAL STUDIES
The efficacy of ORALAIR for the treatment of grass pollen-induced allergic rhinoconjunctivitis was investigated in five double-blind, placebo-controlled clinical trials: four natural field studies and an environmental exposure chamber study.
The natural field studies included these trials, each conducted over a single season (two in adults and one in adolescents and children) and one five-year study (adults). Participants received ORALAIR or placebo daily for four months prior to grass pollen season and throughout grass pollen season.
Study participants reported at least a two grass pollen season history of rhinoconjunctivitis symptoms. For the European studies, subjects had a positive skin prick test to 5-grass pollen extract and positive in vitro testing for timothy grass-specific serum IgE. For the US study, subjects had a positive skin prick test to Timothy grass pollen extract.
With the exception of those with mild intermittent asthma, patients with asthma were excluded. Approximately 16% had asthma at baseline and 65% were polysensitized (i.e., sensitized to the 5-grass pollen allergen extract and at least one other unrelated allergen). Overall, the mean age of study participants was 28 years and 56% were male.
Natural Field Studies
In the natural field studies, efficacy of ORALAIR as immunotherapy to treat symptoms of allergic rhinoconjunctivitis due to the grass pollens included in ORALAIR was assessed via daily recording of symptoms and rescue medication use. The daily Combined Score (CS, range: 0-3) equally weights symptoms and rescue medication use. The daily Rhinoconjunctivitis Total Symptom Score (RTSS, range 0-18) is the total of the six individual symptom scores (sneezing, rhinorrhea, nasal pruritus, nasal congestion, ocular pruritus and watery eyes) each graded by participants on a 0 (no symptoms) to 3 (severe symptoms) scale. The daily Rescue Medication Score (RMS, range 0-3) grades the intake of rescue medication as 0 = absent, 1 = antihistamine, 2 = nasal corticosteroid, 3 = oral corticosteroid. In case of multiple medications, the higher score is retained. Least Squares (LS) means are within-group means adjusted for the covariates in the statistical models (i.e., analyses of covariance for average scores and linear mixed models with repeated measures for daily scores). The Relative Difference is the LS mean difference between ORALAIR and Placebo divided by the LS mean of Placebo, expressed as a percentage.
US Study
In this study, 473 adults aged 18 through 65 years received ORALAIR or placebo, starting approximately four months prior to the expected onset of the grass-pollen season and continuing for the duration of the pollen season. The results of the analysis of the daily Combined Score (CS), daily Rhinoconjunctivitis Total Symptom Score (RTSS), and daily Rescue Medication Score (RMS) are summarized in Table 4.
Table 4. Daily Combined Score (CS), Daily Rhinoconjunctivitis Total Symptom Score (RTSS), and Daily Rescue Medication Score (RMS) during the Grass Pollen Period (US study) Efficacy endpoint ORALAIR
(N=208)
LS* Mean
Placebo
(N=228)
LS Mean
LS Mean
Difference
ORALAIR
- Placebo
Relative Difference Estimate 95% CI Daily CS † 0.32 0.45 -0.13 -28.2% [-43.4%;-13.0%] Daily RTSS ‡ 3.21 4.16 -0.95 -22.9% [-38.2%;-7.5%] Daily RMS ‡ 0.11 0.20 -0.09 -46.5% [-73.9%;-19.2%] * LS: Least Squares
† Primary efficacy analysis
‡ Secondary efficacy analysisEuropean Study
In this study, adults aged 18 to 45 years received one of 3 different doses of 5-grass pollen extract sublingual tablet or placebo. A total of 311 subjects received ORALAIR or placebo starting approximately 4 months prior to the expected onset of the grass pollen season and continuing for the duration of the grass pollen season. The results of the analysis of the daily CS, daily RTSS and daily RMS for ORALAIR (300 IR) are shown in Table 5.
Table 5. Daily Combined Score (CS), Daily Rhinoconjunctivitis Total Symptom Score (RTSS), and Daily Rescue Medication Score (RMS) during the Grass Pollen Period (European study) Efficacy endpoint ORALAIR
(N=136)
LS* MeanPlacebo
(N=148)
LS MeanLS Mean
Difference
ORALAIR
- Placebo
Relative Difference Estimate 95% CI Daily CS 0.50 0.70 -0.21 -29.6% [-43.1%;-16.1%] Daily RTSS 3.48 4.91 -1.44 -29.2% [-43.4%;-15.1%] Daily RMS 0.41 0.59 -0.18 -30.1% [-49.5%;-10.6%] * LS: Least Squares
Long Term Study
In this study, adults received ORALAIR or placebo according to two different treatment regimens. A total of 426 subjects received ORALAIR or placebo starting approximately 4 months prior to the grass pollen season and continuing for the entire season. Subjects were treated for three consecutive grass pollen seasons (Year 1 to Year 3). The primary evaluation was the Year 3 pollen period. Participants then entered two years of immunotherapy-free follow-up (Year 4 and Year 5). The results of the analysis of the daily Combined Score for ORALAIR (4M) for treatment Years 1-3 are summarized in Table 6. Data are insufficient to demonstrate efficacy for one or two years after discontinuation of ORALAIR.
Table 6. Analysis of Daily Combined Score for Each Grass Pollen Period (Long Term study) Year ORALAIR (4M) Placebo LS Mean
Difference
ORALAIR
- Placebo
Relative Difference N LS* Mean N LS Mean Estimate 95% CI Year 1 188 0.56 205 0.67 -0.11 -16.4% [-27.0%;-5.8%] Year 2 160 0.35 172 0.56 -0.21 -38.0% [-53.4%;-22.6%] Year 3 149 0.31 165 0.50 -0.19 -38.3% [-54.7%;-22.0%] * LS: Least Squares
Pediatric Study
In this study, 278 children and adolescents 5 through 17 years of age received ORALAIR or placebo starting approximately 4 months prior to the grass-pollen season and continuing for the duration of the pollen season. The results of the daily CS, daily RTSS, and daily RMS are summarized in Table 7.
Table 7. Daily Combined Score (CS), Daily Rhinoconjunctivitis Total Symptom Score (RTSS), Daily Rescue Medication Score (RMS) during the Grass Pollen Period (Pediatric study) Efficacy endpoint ORALAIR
(N=131)
LS* MeanPlacebo
(N=135)
LS MeanLS Mean
Difference
ORALAIR
- Placebo
Relative Difference Estimate 95% CI Daily CS 0.44 0.63 -0.19 -30.1% [-46.9%;-13.2%] Daily RTSS 2.52 3.63 -1.11 -30.6% [-47.0%;-14.1%] Daily RMS 0.46 0.65 -0.19 -29.5% [-50.9%;-8.0%] * LS: Least Squares
Allergen Environmental Chamber Study
In an allergen environmental chamber study, 89 adults with grass pollen-associated allergic rhinoconjunctivitis were challenged with four of the five grass pollens contained in ORALAIR at baseline and after 4 months of treatment with ORALAIR (n=45) or placebo (n=44). The average Rhinoconjunctivitis Total Symptom Score (RTSS) of each group during the 4 hours of the allergen challenge was assessed; use of rescue medication was not permitted. The results of this study are shown in Table 8.
Table 8. Average Rhinoconjunctivitis Total Symptom Score (RTSS) during Grass Pollen Allergen Challenge in an Environmental Exposure Chamber after 4 months of ORALAIR or placebo Efficacy endpoint ORALAIR
(N=45)
LS* MeanPlacebo
(N=44)
LS MeanLS Mean
Difference
ORALAIR
- Placebo
Relative Difference Estimate 95% CI Average RTSS † 4.88 6.84 -1.97 -28.7% [-43.7%;-13.7%] * LS: Least Squares
† Primary efficacy analysis -
16 HOW SUPPLIED/STORAGE AND HANDLING
ORALAIR is available as a sublingual tablet equivalent to 100 IR and 300 IR of five grass mixed pollens allergen extract.
Description NDC Number Children and
Adolescents
Sample Kit
(5 to 17 years of age)One box of the 100 IR
Starter Pack
Two boxes of the 300 IRSample Packs
NDC: 59617-0020-1 Adult
Sample Kit
(18 to 65 years of age)One box of 300 IR Starter
Pack
Two boxes of 300 IRSample Packs
NDC: 59617-0025-1 Children and
Adolescents
Starter Pack
(5 to 17 years of age)1 blister pack of three 100
IR tablets
NDC: 59617-0010-1 Adult
Starter Pack
(18 to 65 years of age)1 blister pack of three 300
IR tablets
NDC: 59617-0016-1 Sample Pack 1 blister pack of three 300
IR tablets
NDC: 59617-0015-3 Commercial Pack 1 blister pack of thirty 300
IR tablets
NDC: 59617-0015-2 Storage: Store at controlled room temperature (20°C-25°C/68°F-77°F); excursions permitted to 15-30°C (59-86°F). Protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
- Keep ORALAIR out of the reach of children.
- Inform patients that ORALAIR is used for sublingual immunotherapy for the treatment of grass pollen-induced allergic rhinitis with or without conjunctivitis and is not indicated for the immediate relief of allergy symptoms.
Severe Allergic Reactions
- Advise patients that ORALAIR may cause systemic allergic reactions, including anaphylactic reactions, and severe local allergic reactions [ See Warnings and Precautions (5.1)].
- Educate patients about the signs and symptoms of a severe systemic allergic reaction and a severe local allergic reaction. The signs and symptoms of a severe allergic reaction may include: syncope, dizziness, hypotension, tachycardia, dyspnea, wheezing, bronchospasm, chest discomfort, cough, abdominal pain, vomiting, diarrhea, rash, pruritus, flushing, and urticaria [ See Warnings and Precautions (5.2)].
- Ensure that patients have injectable epinephrine available and are appropriately trained in its use. Instruct patients who experience a severe allergic reaction to seek immediate medical care, discontinue therapy, and resume treatment only at the instruction of a physician [ See Warnings and Precautions (5.2)].
- Inform the patient that the first dose of ORALAIR is administered in a healthcare setting under the supervision of a physician and s/he will be monitored for at least 30 minutes to watch for signs and symptoms of a severe systemic or a severe local allergic reaction [ See Dosage and Administration (2.2)].
- Inform parents/guardians that ORALAIR should be administered to children only under adult supervision [ See Dosage and Administration (2.2)].
- Because of the risk of eosinophilic esophagitis, instruct patients with severe or persistent symptoms of esophagitis to discontinue ORALAIR and to contact their healthcare professional. [ See Warnings and Precautions (5.3)]
Asthma
- Instruct patients with asthma that if they have difficulty breathing or if asthma becomes difficult to control, they are to stop taking ORALAIR and contact their healthcare professional immediately [ See Warnings AND Precautions (5.3)].
Administration Instructions
- Instruct patients to carefully remove the ORALAIR tablet from the blister just prior to dosing and to take the sublingual tablet immediately by placing it under the tongue where it will dissolve. Also instruct patients to avoid swallowing for about 1 minute, to wash their hands after handling the tablet, and to avoid food or beverages for 5 minutes after taking the tablet [ See Dosage and Administration (2.2)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
ORALAIR ® (OR-AL-AIR): (Sweet Vernal, Orchard, Perennial Rye, Timothy, and Kentucky Blue Grass Mixed Pollens Allergen Extract)
Carefully read this Medication Guide before you or your child start taking ORALAIR and each time you get a refill. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment. Talk with your doctor or pharmacist if there is something you do not understand or you want to learn more about ORALAIR.
What is the Most Important Information I Should Know about ORALAIR?
ORALAIR can cause severe allergic reactions that may be life-threatening. Symptoms of allergic reactions to ORALAIR include:
- Trouble breathing
- Throat tightness or swelling
- Trouble swallowing or speaking
- Dizziness or fainting
- Rapid or weak heartbeat
- Severe stomach cramps or pain, vomiting, or diarrhea
- Severe flushing or itching of the skin
If any of these symptoms occur, stop taking ORALAIR and immediately seek medical care.
For home administration of ORALAIR, your doctor should prescribe auto-injectable epinephrine for you to keep at home for treating a severe reaction, should one occur. Your doctor will train and instruct you on the proper use of auto-injectable epinephrine.
What is ORALAIR
ORALAIR is a prescription medicine used for sublingual (under the tongue) immunotherapy prescribed to treat sneezing, runny or itchy nose, nasal congestion or itchy and watery eyes due to allergy to these grass pollens. ORALAIR may be prescribed for persons 5 to 65 years of age whose doctor has confirmed are allergic to any of the grass pollens contained in ORALAIR.
ORALAIR is taken for about four months before the expected start of the grass pollen season and is continued throughout the grass pollen season.
ORALAIR is NOT a medication that gives immediate relief of allergy symptoms.
Who Should Not Take ORALAIR
You or your child should not take ORALAIR if:
- You or your child has severe, unstable, or uncontrolled asthma
- You or your child had a severe allergic reaction in the past that included any of these symptoms:
- Trouble breathing
- Dizziness or fainting
- Rapid or weak heartbeat
- You or your child has ever had difficulty with breathing due to swelling of the throat or upper airway after using any sublingual immunotherapy before.
- You or your child has ever been diagnosed with eosinophilic esophagitis.
- You or your child is allergic to any of the inactive ingredients contained in ORALAIR
- The inactive ingredients contained in ORALAIR are: mannitol, microcrystalline cellulose, croscarmellose sodium, colloidal
anhydrous silica, magnesium stearate and lactose monohydrate
What Should I Tell My Doctor Before Taking ORALAIR
Your doctor may decide that ORALAIR is not the best course of therapy if:
- You or your child has asthma, depending on how severe it is
- You or your child suffers from lung disease such as chronic obstructive pulmonary disease (COPD)
- You or your child suffers from heart disease such as coronary artery disease, an irregular heart rhythm, or you have hypertension that is not well controlled.
- You or your daughter is pregnant, plans to become pregnant during the time you will be taking ORALAIR, or is breast-feeding.
- You or your child is unable or unwilling to administer auto-injectable epinephrine to treat a severe allergic reaction to ORALAIR
- You or your child is taking certain medicines that enhance the likelihood of a severe reaction, or interfere with the treatment of a severe reaction. These medicines include:
- beta blockers and alpha-blockers (prescribed for high blood pressure)
- cardiac glycosides (prescribed for heart failure or problems with heart rhythm)
- diuretics (prescribed for heart conditions and high blood pressure)
- ergot alkaloids (prescribed for migraine headache)
- monoamine oxidase inhibitors or tricyclic antidepressants (prescribed for depression)
- thyroid hormone (prescribed for low thyroid activity);
You should tell your doctor if you or your child is taking or has recently taken any other medicines, including medicines obtained without a prescription and herbal supplements. Keep a list of them and show it to your doctor and pharmacist each time you get a new supply of ORALAIR. Ask your doctor or pharmacist for advice before taking ORALAIR.
Are there any reasons to stop taking ORALAIR?
Stop ORALAIR and contact your doctor if you or your child:
- has any type of a serious allergic reaction
- develops throat tightness or swelling of the tongue or throat that causes trouble speaking, breathing or swallowing after taking ORALAIR
- has trouble breathing or asthma or another breathing condition that gets worse
- experiences dizziness or fainting
- develops rapid or weak heartbeat
- experiences severe stomach cramps or pain, vomiting, or diarrhea
- develops severe flushing or itching of the skin
- has heartburn, difficulty swallowing, pain with swallowing, or chest pain that does not go away or worsens
- has any mouth surgery procedures (such as tooth removal), develops any mouth infections, ulcers or cuts in the mouth or throat
How should I take ORALAIR?
Take ORALAIR exactly as your doctor tells you.
ORALAIR is a prescription medicine that is placed under the tongue.
- Remove the ORALAIR tablet from the blister just prior to dosing.
- Place the ORALAIR tablet immediately under the tongue until complete dissolution for at least 1 minute before swallowing.
- Do not take ORALAIR with food or beverage. Food and beverage should not be taken for the following 5 minutes.
- Wash hands after handling the tablet.
Take the first tablet of ORALAIR in your doctor's office. After taking the first tablet, you or your child will be observed for at least 30 minutes for symptoms of a serious allergic reaction.
- The first dose for children will be one 100 IR tablet.
- The first dose for adults will be one 300 IR tablet.
If you or your child tolerates the first dose of ORALAIR, you or your child will continue daily ORALAIR therapy at home.
- The first dose at home for children is two 100 IR tablets.
- The first dose at home for adults is one 300 IR tablet.
- After the first dose at home, the dose for children and adults is one 300 IR tablet each day.
Children should be given each dose of ORALAIR by an adult who will watch for any symptoms of a serious allergic reaction.
Take ORALAIR as prescribed by your doctor until the end of the treatment course. If you forget to take ORALAIR, do not take a double dose. Take the next dose at your normal scheduled time the next day. If you don't take ORALAIR for more than one day, contact your health provider before restarting.
What are the possible side effects of ORALAIR?
In children and adults, the most commonly reported side effects were itching of the mouth, lips, tongue or throat. These side effects, by themselves, are not dangerous or life-threatening.
ORALAIR can cause severe allergic reactions that may be life-threatening. Symptoms of allergic reactions to ORALAIR include:
- Trouble breathing
- Throat tightness or swelling
- Trouble swallowing or speaking
- Dizziness or fainting
- Rapid or weak heartbeat
- Severe stomach cramps or pain, vomiting, or diarrhea
- Severe flushing or itching of the skin
For additional information on the possible side effects of ORALAIR, talk with your doctor or pharmacist. You may report side effects to the US Food and Drug Administration (FDA) at 1-800-FDA-1088 or www.fda.gov/medwatch.
How should I store ORALAIR?
Keep ORALAIR out of the reach of children.
Throw away any unused ORALAIR after the expiration date which is stated on the carton and blister pack after "EXP."
Store ORALAIR in a dry place at room temperature, 20°C to 25°C (68°F to 77°F), in the original package.
General information about ORALAIR
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ORALAIR for a condition for which it was not prescribed. Do not give ORALAIR to other people, even if they have the same symptoms. It may harm them.
This Medication Guide summarizes the most important information about ORALAIR. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about ORALAIR that was written for healthcare professionals. For more information go to www.ORALAIR.com or call Greer Laboratories at 1-855-752-5046.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
ORALAIR ® is a registered trademark of Stallergenes SAS
Manufactured by:
Stallergenes SAS
Antony, 92183, France
U.S. License # 1893Distributed by:
GREER Laboratories, Inc.
Lenoir, N.C. 28645 -
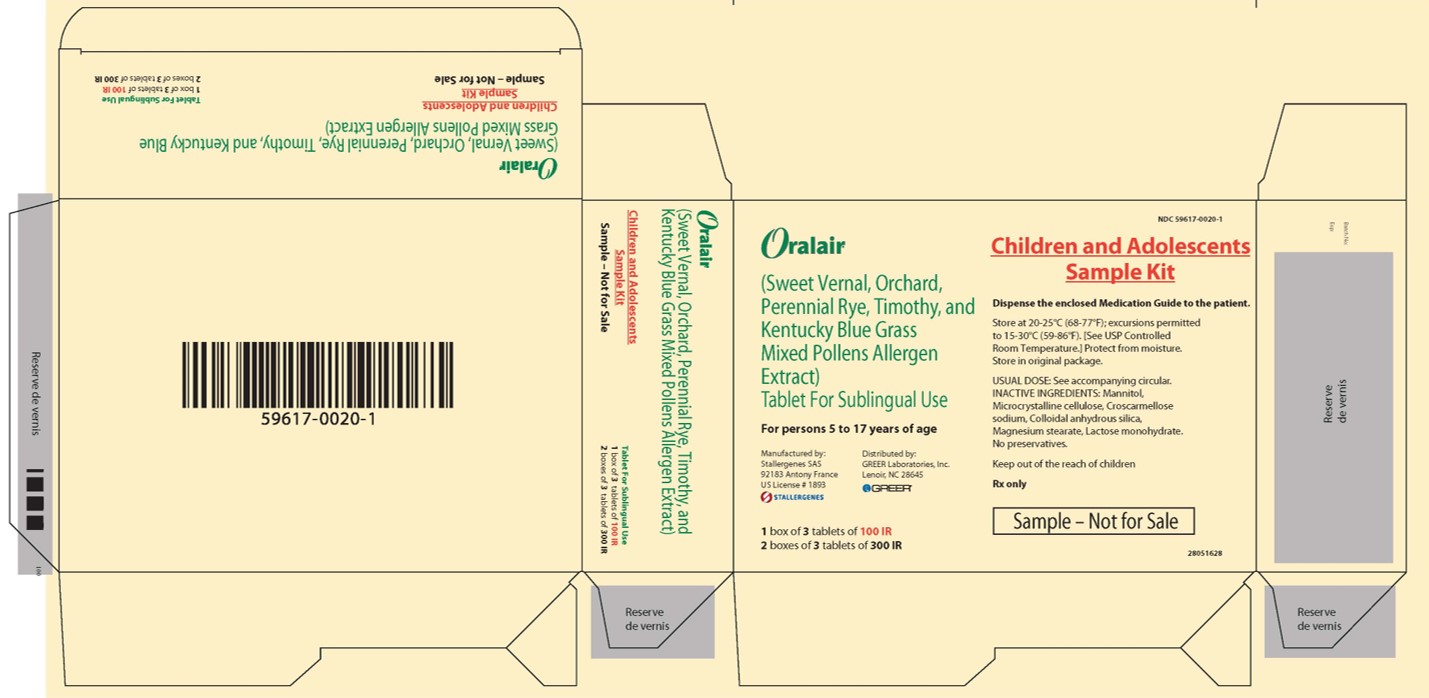
PRINCIPAL DISPLAY PANEL - CHILDREN KIT CARTON
Oralair ®
(Sweet Vernal, Orchard,
Perennial Rye, Timothy, and
Kentucky Blue Grass
Mixed Pollens Allergen
Extract)Tablet For Sublingual Use
For persons 5 to 17 years of age
Manufactured by:
Stallergenes SAS
92183 Antony France
US License # 1893
STALLERGENESDistributed by:
GREER Laboratories, Inc.
Lenoir, NC 28645
GREER ®1 box of 3 tablets of 100 IR
2 boxes of 3 tablets of 300 IRNDC: 59617-0020-1
Children and Adolescents
Sample KitDispense the enclosed Medication Guide to the patient.
Store at 20-25°C (68-77°F); excursions permitted
to 15-30°C (59-86°F). [See USP Controlled
Room Temperature.] Protect from moisture.
Store in original package.USUAL DOSE: See accompanying circular.
INACTIVE INGREDIENTS: Mannitol,
Microcrystalline cellulose, Croscarmellose
sodium, Colloidal anhydrous silica,
Magnesium stearate, Lactose monohydrate.
No preservatives.Keep out of the reach of children
Rx only
Sample – Not for Sale
41000
-
PRINCIPAL DISPLAY PANEL - ADULT KIT CARTON
Oralair ®
(Sweet Vernal, Orchard,
Perennial Rye, Timothy, and
Kentucky Blue Grass
Mixed Pollens Allergen
Extract)
Tablet For Sublingual UseFor persons 18 to 65 years of age
Manufactured by:
Stallergenes SAS
92183 Antony France
US License # 1893
STALLERGENESDistributed by:
GREER Laboratories, Inc.
Lenoir, NC 28645
GREER ®3 boxes of 3 tablets of 300 IR
NDC: 59617-0025-1
Adult
Sample KitDispense the enclosed Medication Guide to the patient.
Store at 20-25°C (68-77°F); excursions permitted
to 15-30°C (59-86°F). [See USP Controlled
Room Temperature.] Protect from moisture.
Store in original package.USUAL DOSE: See accompanying circular.
INACTIVE INGREDIENTS: Mannitol,
Microcrystalline cellulose, Croscarmellose
sodium, Colloidal anhydrous silica,
Magnesium stearate, Lactose monohydrate.
No preservatives.Keep out of the reach of children
Rx only
Sample – Not for Sale
41004

-
PRINCIPAL DISPLAY PANEL - 300 IR 30 TABLET BLISTER PACK BOX
Oralair ®
300 IR
(Sweet Vernal, Orchard,
Perennial Rye, Timothy, and
Kentucky Blue Grass
Mixed Pollens Allergen
Extract)
Tablet For Sublingual UseFor Persons 5 to 65 years of age
Manufactured by:
Stallergenes SAS
92183 Antony France
US License # 1893
STALLERGENESDistributed by:
GREER Laboratories, Inc.
Lenoir, NC 28645
GREER ®1 blister of 30 tablets of 300 IR
NDC: 59617-0015-2
Dispense the enclosed Medication Guide
to the patientStore at 20-25°C (68-77°F); excursions
permitted to 15-30°C (59-86°F).
[See USP Controlled Room Temperature.]
Protect from moisture. Store in original
package.USUAL DOSE: See accompanying circular.
INACTIVE INGREDIENTS: Mannitol,
Microcrystalline cellulose, Croscarmellose
sodium, Colloidal anhydrous silica,
Magnesium stearate, Lactose monohydrate.
No preservatives.Keep out of the reach of children
Rx only
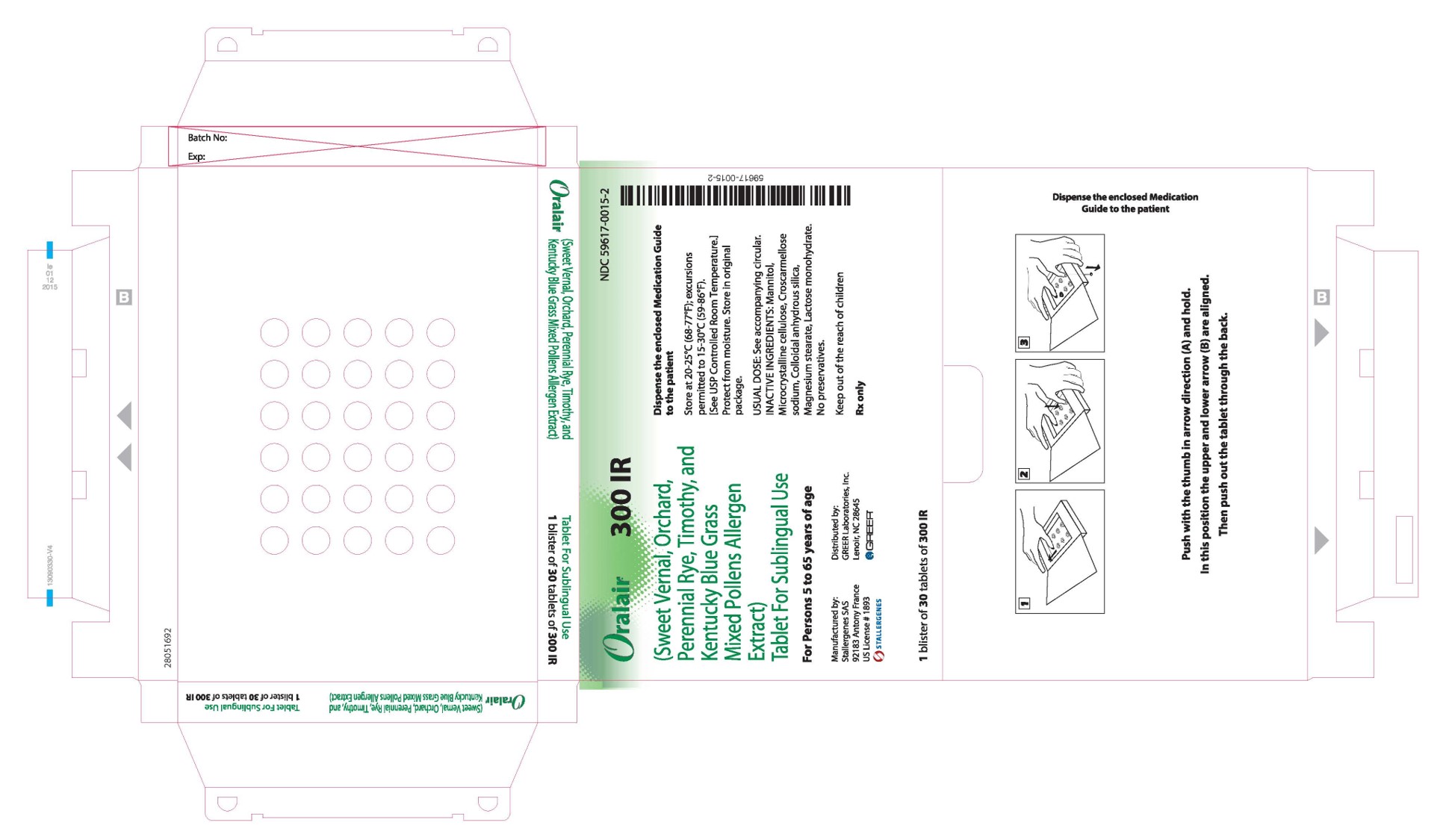
-
PRINCIPAL DISPLAY PANEL - 300 IR 3 TABLET BLISTER PACK BOX
Oralair ®
300 IR(Sweet Vernal, Orchard,
Perennial Rye, Timothy, and
Kentucky Blue Grass
Mixed Pollens Allergen
Extract)
Tablet For Sublingual UseFor Persons 18 to 65 years of age
Manufactured by:
Stallergenes SAS
92183 Antony France
US License # 1893
STALLERGENESDistributed by:
GREER Laboratories, Inc.
Lenoir, NC 28645
GREER ®1 blister of 3 tablets of 300 IR
NDC: 59617-0016-1
Adult
Starter PackDispense the enclosed Medication Guide
to the patientStore at 20-25°C (68-77°F); excursions
permitted to 15-30°C (59-86°F).
[See USP Controlled Room Temperature.]
Protect from moisture. Store in original
package.USUAL DOSE: See accompanying circular.
INACTIVE INGREDIENTS: Mannitol,
Microcrystalline cellulose, Croscarmellose
sodium, Colloidal anhydrous silica,
Magnesium stearate, Lactose monohydrate.
No preservatives.Keep out of the reach of children
Rx only
Sample – Not for Sale
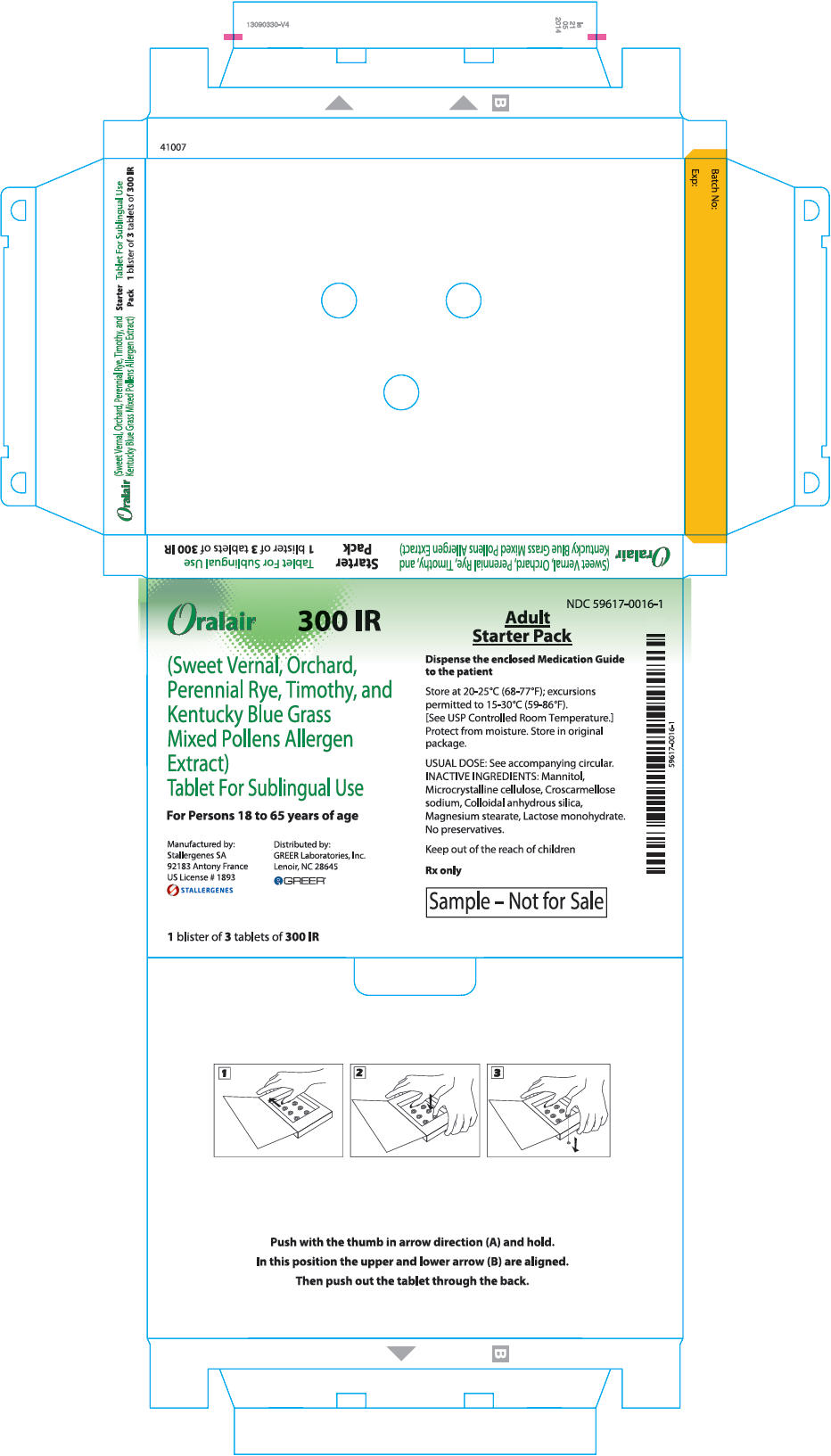
-
PRINCIPAL DISPLAY PANEL - 100 IR 3 TABLET BLISTER PACK BOX
Oralair ®
100 IR(Sweet Vernal, Orchard,
Perennial Rye, Timothy, and
Kentucky Blue Grass
Mixed Pollens Allergen
Extract)
Tablet For Sublingual UseFor Persons 5 to 17 years of age
Manufactured by:
Stallergenes SAS
92183 Antony France
US License # 1893
STALLERGENESDistributed by:
GREER Laboratories, Inc.
Lenoir, NC 28645
GREER ®1 blister of 3 tablets of 100 IR
NDC: 59617-0010-1
Children and Adolescents
Starter PackDispense the enclosed Medication Guide
to the patientStore at 20-25°C (68-77°F); excursions
permitted to 15-30°C (59-86°F).
[See USP Controlled Room Temperature.]
Protect from moisture. Store in original
package.USUAL DOSE: See accompanying circular.
INACTIVE INGREDIENTS: Mannitol,
Microcrystalline cellulose, Croscarmellose
sodium, Colloidal anhydrous silica,
Magnesium stearate, Lactose monohydrate.
No preservatives.Keep out of the reach of children
Rx only
Sample – Not for Sale
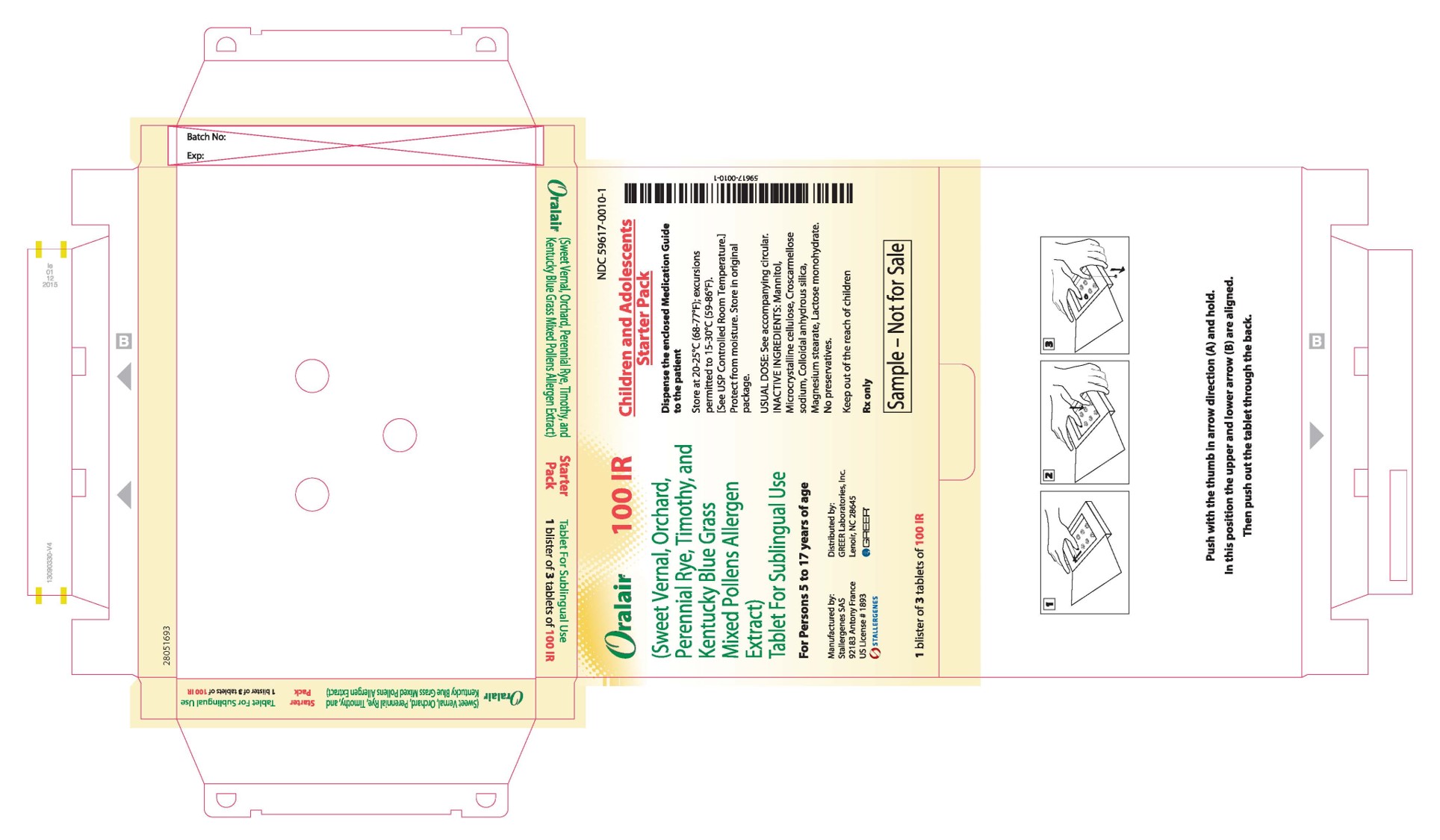
-
INGREDIENTS AND APPEARANCE
ORALAIR
anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phelum pratense pollen, and poa pratensis pollen kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 82454-0025 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82454-0025-1 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 BOX 3 Part 2 3 BOX 6 Part 1 of 2 ORALAIR 300 IR
anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phelum pratense pollen, and poa pratensis pollen tablet, orally disintegratingProduct Information Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHLEUM PRATENSE POLLEN (UNII: 65M88RW2EG) (PHLEUM PRATENSE TOP - UNII:S7PW24BX20, PHLEUM PRATENSE POLLEN - UNII:65M88RW2EG) PHLEUM PRATENSE POLLEN 300 [IR] ANTHOXANTHUM ODORATUM POLLEN (UNII: 2KIK19R45Y) (ANTHOXANTHUM ODORATUM POLLEN - UNII:2KIK19R45Y) ANTHOXANTHUM ODORATUM POLLEN 300 [IR] DACTYLIS GLOMERATA POLLEN (UNII: 83N78IDA7P) (DACTYLIS GLOMERATA POLLEN - UNII:83N78IDA7P) DACTYLIS GLOMERATA POLLEN 300 [IR] POA PRATENSIS POLLEN (UNII: SCB8J7LS3T) (POA PRATENSIS POLLEN - UNII:SCB8J7LS3T) POA PRATENSIS POLLEN 300 [IR] LOLIUM PERENNE POLLEN (UNII: 4T81LB52R0) (LOLIUM PERENNE POLLEN - UNII:4T81LB52R0) LOLIUM PERENNE POLLEN 300 [IR] Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (Slightly speckled white to beige) Score no score Shape ROUND Size 6mm Flavor Imprint Code 300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125471 04/30/2014 Part 2 of 2 ORALAIR 300 IR
anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phelum pratense pollen, and poa pratensis pollen tablet, orally disintegratingProduct Information Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHLEUM PRATENSE POLLEN (UNII: 65M88RW2EG) (PHLEUM PRATENSE TOP - UNII:S7PW24BX20, PHLEUM PRATENSE POLLEN - UNII:65M88RW2EG) PHLEUM PRATENSE POLLEN 300 [IR] ANTHOXANTHUM ODORATUM POLLEN (UNII: 2KIK19R45Y) (ANTHOXANTHUM ODORATUM POLLEN - UNII:2KIK19R45Y) ANTHOXANTHUM ODORATUM POLLEN 300 [IR] DACTYLIS GLOMERATA POLLEN (UNII: 83N78IDA7P) (DACTYLIS GLOMERATA POLLEN - UNII:83N78IDA7P) DACTYLIS GLOMERATA POLLEN 300 [IR] POA PRATENSIS POLLEN (UNII: SCB8J7LS3T) (POA PRATENSIS POLLEN - UNII:SCB8J7LS3T) POA PRATENSIS POLLEN 300 [IR] LOLIUM PERENNE POLLEN (UNII: 4T81LB52R0) (LOLIUM PERENNE POLLEN - UNII:4T81LB52R0) LOLIUM PERENNE POLLEN 300 [IR] Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (Slightly speckled white to beige) Score no score Shape ROUND Size 6mm Flavor Imprint Code 300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125471 04/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125471 04/30/2014 ORALAIR 300 IR
anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phleum pratense pollen, and poa pratensis pollen tablet, orally disintegratingProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 82454-0015 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DACTYLIS GLOMERATA POLLEN (UNII: 83N78IDA7P) (DACTYLIS GLOMERATA POLLEN - UNII:83N78IDA7P) DACTYLIS GLOMERATA POLLEN 300 [IR] ANTHOXANTHUM ODORATUM POLLEN (UNII: 2KIK19R45Y) (ANTHOXANTHUM ODORATUM POLLEN - UNII:2KIK19R45Y) ANTHOXANTHUM ODORATUM POLLEN 300 [IR] LOLIUM PERENNE POLLEN (UNII: 4T81LB52R0) (LOLIUM PERENNE POLLEN - UNII:4T81LB52R0) LOLIUM PERENNE POLLEN 300 [IR] PHLEUM PRATENSE POLLEN (UNII: 65M88RW2EG) (PHLEUM PRATENSE TOP - UNII:S7PW24BX20) PHLEUM PRATENSE POLLEN 300 [IR] POA PRATENSIS POLLEN (UNII: SCB8J7LS3T) (POA PRATENSIS POLLEN - UNII:SCB8J7LS3T) POA PRATENSIS POLLEN 300 [IR] Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white ((Slightly speckled white to beige)) Score no score Shape ROUND Size 6mm Flavor Imprint Code 300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82454-0015-3 1 in 1 BOX 1 3 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 82454-0015-2 30 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125471 04/30/2014 ORALAIR
anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phelum pratense pollen, and poa pratensis pollen kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 82454-0020 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82454-0020-1 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 BOX 6 Part 2 3 BOX 3 Part 1 of 2 ORALAIR 300 IR
anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phelum pratense pollen, and poa pratensis pollen tablet, orally disintegratingProduct Information Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHLEUM PRATENSE POLLEN (UNII: 65M88RW2EG) (PHLEUM PRATENSE TOP - UNII:S7PW24BX20, PHLEUM PRATENSE POLLEN - UNII:65M88RW2EG) PHLEUM PRATENSE POLLEN 300 [IR] ANTHOXANTHUM ODORATUM POLLEN (UNII: 2KIK19R45Y) (ANTHOXANTHUM ODORATUM POLLEN - UNII:2KIK19R45Y) ANTHOXANTHUM ODORATUM POLLEN 300 [IR] DACTYLIS GLOMERATA POLLEN (UNII: 83N78IDA7P) (DACTYLIS GLOMERATA POLLEN - UNII:83N78IDA7P) DACTYLIS GLOMERATA POLLEN 300 [IR] POA PRATENSIS POLLEN (UNII: SCB8J7LS3T) (POA PRATENSIS POLLEN - UNII:SCB8J7LS3T) POA PRATENSIS POLLEN 300 [IR] LOLIUM PERENNE POLLEN (UNII: 4T81LB52R0) (LOLIUM PERENNE POLLEN - UNII:4T81LB52R0) LOLIUM PERENNE POLLEN 300 [IR] Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (Slightly speckled white to beige) Score no score Shape ROUND Size 6mm Flavor Imprint Code 300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125471 04/30/2014 Part 2 of 2 ORALAIR 100 IR
anthoxanthum odoratum pollen, dactylis glomerata pollen, lolium perenne pollen, phelum pratense pollen, and poa pratensis pollen tablet, orally disintegratingProduct Information Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHLEUM PRATENSE POLLEN (UNII: 65M88RW2EG) (PHLEUM PRATENSE TOP - UNII:S7PW24BX20, PHLEUM PRATENSE POLLEN - UNII:65M88RW2EG) PHLEUM PRATENSE POLLEN 100 [IR] LOLIUM PERENNE POLLEN (UNII: 4T81LB52R0) (LOLIUM PERENNE POLLEN - UNII:4T81LB52R0) LOLIUM PERENNE POLLEN 100 [IR] ANTHOXANTHUM ODORATUM POLLEN (UNII: 2KIK19R45Y) (ANTHOXANTHUM ODORATUM POLLEN - UNII:2KIK19R45Y) ANTHOXANTHUM ODORATUM POLLEN 100 [IR] DACTYLIS GLOMERATA POLLEN (UNII: 83N78IDA7P) (DACTYLIS GLOMERATA POLLEN - UNII:83N78IDA7P) DACTYLIS GLOMERATA POLLEN 100 [IR] POA PRATENSIS POLLEN (UNII: SCB8J7LS3T) (POA PRATENSIS POLLEN - UNII:SCB8J7LS3T) POA PRATENSIS POLLEN 100 [IR] Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (Slightly speckled white to beige) Score no score Shape ROUND Size 6mm Flavor Imprint Code 100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125471 04/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125471 04/30/2014 Labeler - CENEXI HSC (268155718) Establishment Name Address ID/FEI Business Operations CENEXI - Osny 263382610 manufacture(82454-0015, 82454-0020, 82454-0025)
Trademark Results [Oralair]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ORALAIR 85807637 4593632 Live/Registered |
STALLERGENES 2012-12-20 |
 ORALAIR 79012205 3194354 Live/Registered |
STALLERGENES 2005-06-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.