EVOMELA (melphalan) for injection, for intravenous use. These highlights do not include all the information needed to use EVOMELA ® safely and effectively. See full prescribing information for EVOMELA. Initial U.S. Approval: 1964
EVOMELA by
Drug Labeling and Warnings
EVOMELA by is a Prescription medication manufactured, distributed, or labeled by CENEXI - BLA, Cenexi-Laboratoires Thissen S.A. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EVOMELA- melphalan injection, powder, lyophilized, for solution
CENEXI - BLA
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONEVOMELA (melphalan) for injection, for intravenous use.
These highlights do not include all the information needed to use EVOMELA ® safely and effectively. See full prescribing information for EVOMELA. Initial U.S. Approval: 1964
WARNING: SEVERE BONE MARROW SUPPRESSION, HYPERSENSITIVITY, AND LEUKEMOGENICITY
|
FULL PRESCRIBING INFORMATION
WARNING: SEVERE BONE MARROW SUPPRESSION, HYPERSENSITIVITY, and LEUKEMOGENICITY
-
Severe bone marrow suppression with resulting infection or bleeding may occur. Controlled trials comparing intravenous (IV) melphalan to oral melphalan have shown more myelosuppression with the IV formulation. Monitor hematologic laboratory parameters.
[see Warnings and Precautions (
5.1)]
-
Hypersensitivity reactions, including anaphylaxis, have occurred in approximately 2% of patients who received the IV formulation of melphalan. Discontinue treatment with Evomela for serious hypersensitivity reactions.
[see Warnings and Precautions (
5.4)]
- Melphalan produces chromosomal aberrations in vitro and in vivo. Evomela should be considered potentially leukemogenic in humans. [see Warnings and Precautions ( 5.5)]
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Conditioning Treatment
The recommended dose of Evomela for conditioning treatment is 100 mg/m 2/day administered over 30 minutes by intravenous infusion for 2 consecutive days (Day -3 and Day -2) prior to autologous stem cell transplantation (ASCT, Day 0). For patients who weigh more than 130% of their ideal body weight, body surface area should be calculated based on adjusted ideal body weight.
Administer prophylactic antiemetics [see Warnings and Precautions ( 5.2)] .
2.2 Preparation and Administration
Evomela is a hazardous drug. Follow applicable special handling and disposal procedures 1.
Evomela is light sensitive. Retain in original carton until use.
Do not mix Evomela with other melphalan hydrochloride for injection drug products.
Reconstitution and Infusion Instructions:
1. Use normal saline solution (0.9% Sodium Chloride Injection, USP) (8.6 mL as directed) to reconstitute Evomela and make a 50 mg/10 mL (5 mg/ mL) nominal concentration of melphalan.
The reconstituted Evomela drug product is stable for 24 hours at refrigerated temperature (5 oC) without any precipitation due to the high solubility.
The reconstituted Evomela drug product is stable for 1 hour at room temperature.
2. Calculate the required volume of Evomela needed for a patient’s dose and withdraw that volume from the vial(s).
3. Add the required volume of Evomela to the appropriate volume of 0.9% Sodium Chloride Injection, USP to a final concentration of 0.45 mg/mL.
The Evomela admixture solution is stable for 4 hours at room temperature in addition to the 1 hour following reconstitution.
4. Infuse over 30 minutes via an injection port or central venous catheter.
Evomela may cause local tissue damage should extravasation occur. Do not administer by direct injection into a peripheral vein. Administer Evomela by injecting slowly into a fast-running IV infusion via a central venous access line.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
3 DOSAGE FORMS AND STRENGTHS
For injection: 50 mg, white to off-white lyophilized powder in single-dose vial for reconstitution (after reconstitution the solution is clear and colorless to light yellow). Each vial contains 50 mg melphalan free base equivalent to 56 mg melphalan hydrochloride.
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
For patients receiving Evomela as part of a conditioning regimen, myeloablation occurs in all patients. Do not begin the conditioning regimen if a stem cell product is not available for rescue. Monitor complete blood counts, provide supportive care for infections, anemia and thrombocytopenia until there is adequate hematopoietic recovery.
5.2 Gastrointestinal Toxicity
For patients receiving Evomela as part of a conditioning regimen, nausea, vomiting, mucositis, and diarrhea may occur in over 50% of patients. Use prophylactic antiemetic medication. Provide supportive care for nausea, vomiting, diarrhea, and mucositis. The frequency of grade 3/4 mucositis in clinical studies was 13%. Provide nutritional support and analgesics for patients with severe mucositis. [see Dosage and Administration ( 2.1) and Adverse Reactions ( 6.1)] .
5.3 Hepatotoxicity
Hepatic disorders ranging from abnormal liver function tests to clinical manifestations such as hepatitis and jaundice have been reported after treatment with melphalan. Hepatic veno-occlusive disease has also been reported. Monitor liver chemistries.
5.4 Hypersensitivity
Acute hypersensitivity reactions, including anaphylaxis, have occurred in approximately 2% of patients who received an intravenous formulation of melphalan. Symptoms may include urticaria, pruritus, edema, and skin rashes and, in some patients, tachycardia, bronchospasm, dyspnea, and hypotension. Discontinue treatment with Evomela for serious hypersensitivity reactions.
5.5 Secondary Malignancies
Melphalan has been shown to cause chromatid or chromosome damage in humans. Secondary malignancies such as myeloproliferative syndrome or acute leukemia have been reported in multiple myeloma patients treated with melphalan-containing chemotherapy regimens. The potential benefit of Evomela therapy must be considered against the possible risk of the induction of a secondary malignancy.
5.6 Embryo-Fetal Toxicity
Based on its mechanism of action, Evomela can cause fetal harm when administered to a pregnant woman. Melphalan is genotoxic, targets actively dividing cells, and was embryolethal and teratogenic in rats. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Evomela and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Evomela and for 3 months after the last dose [see Use in Specific Populations ( 8.1, 8.3)] .
5.7 Infertility
Melphalan-based chemotherapy regimens have been reported to cause suppression of ovarian function in premenopausal women, resulting in persistent amenorrhea in approximately 9% of patients. Reversible or irreversible testicular suppression has also been reported [see Use in Specific Populations ( 8.3)].
6 ADVERSE REACTIONS
The following serious adverse reactions are described in more detail in other sections of the prescribing information.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of Evomela may not reflect the rates observed in practice.
The most common adverse reactions observed in at least 50% of patients with multiple myeloma treated with Evomela were neutrophil count decreased, white blood cell count decreased, lymphocyte count decreased, platelet count decreased, diarrhea, nausea, fatigue, hypokalemia, anemia, and vomiting.
Myeloablative Conditioning in Multiple Myeloma Patients Undergoing ASCT
The safety of Evomela was evaluated in 61 patients with multiple myeloma in a single arm clinical trial in which patients were administered Evomela at a dosage of 100 mg/m 2/day administered over ~30 minutes (range: 24-48 minutes) by intravenous (IV) infusion for 2 consecutive days (Day -3 and Day -2) prior to autologous stem cell transplant (ASCT, Day 0). [see Clinical Studies ( 14.1)].
Table 1 summarizes the adverse reactions from the single-arm trial in patients with multiple myeloma. Severe myelosuppression is expected and these adverse reactions are not listed below.
|
Adverse Reactions |
Number (%) of Patients (N=61) |
|
|
All Grades |
Grade 3or 4 |
|
| All Adverse Reactions |
61 |
61 |
| Diarrhea |
57 (93%) |
2 (3%) |
| Nausea |
55 (90%) |
1 (2%) |
| Fatigue |
47 (77%) |
1 (2%) |
| Hypokalemia |
45 (74%) |
17 (28%) |
| Vomiting |
39 (64%) |
0 (0%) |
| Hypophosphatemia |
30 (49%) |
29 (2%) |
| Decreased Appetite |
30 (49%) |
0 (0%) |
| Pyrexia |
29 (48%) |
2 (3%) |
| Constipation |
29 (48%) |
0 (0%) |
| Febrile Neutropenia |
25 (41%) |
17 (28%) |
| Mucosal Inflammation |
23 (38%) |
6 (10%) |
| Dizziness |
23 (38%) |
0 (0%) |
| Edema Peripheral |
20 (33%) |
0 (0%) |
| Stomatitis |
17 (28%) |
3 (5%) |
| Abdominal Pain |
17 (28%) |
0 (0%) |
| Dysgeusia |
17 (28%) |
0 (0%) |
| Dyspepsia |
16 (26%) |
0 (0%) |
Serious Adverse Reactions
Twelve (20%) patients experienced a treatment emergent serious adverse reaction while on study. The most common serious adverse reactions (>1 patient, 1.6%) were pyrexia, hematochezia, febrile neutropenia, and renal failure. Treatment-related serious adverse reactions reported in >1 patient were pyrexia (n=2, 3%), febrile neutropenia (n=2, 3%), and hematochezia (n=2, 3%).
7 DRUG INTERACTIONS
No formal drug interaction studies have been conducted. The development of severe renal impairment has been reported in patients treated with a single dose of intravenous melphalan 140-250 mg/m 2 followed by standard oral doses of cyclosporine. Intravenous melphalan may also reduce the threshold for BCNU lung toxicity.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, Evomela can cause fetal harm when administered to a pregnant woman, including teratogenicity and/or embryo-fetal lethality [see Clinical Pharmacology ( 12.1)] . Melphalan is a genotoxic drug and can cause chromatid or chromosome damage in humans [see Nonclinical Toxicology ( 13.1)] . In animal studies, melphalan was embryolethal and teratogenic in rats at doses below the recommended clinical doses [see Data]. Advise a pregnant woman of the potential risk to a fetus..
The background risk of major birth defects and miscarriage for the indicated populations are unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
Data
Animal Data
Adequate animal studies have not been conducted with intravenous melphalan. Melphalan was embryolethal and teratogenic in rats following oral administration of 6 to 18 mg/m 2/day for 10 days (0.06 to 0.18 times the highest recommended clinical dose of 100 mg/m 2/day) and intraperitoneal administration of 18 mg/m 2 (0.18 times the highest recommended clinical dose). Malformations resulting from melphalan administration included alterations of the brain (underdevelopment, deformation, meningocele, and encephalocele) and eye (anophthalmia and microphthalmos), reduction of the mandible and tail, and hepatocele (exomphaly).
8.2 Lactation
Risk Summary
It is not known whether melphalan is present in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing children from melphalan, breastfeeding is not recommended during treatment with Evomela and for one week after the last dose.
8.3 Females and Males of Reproductive Potential
Evomela can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)] .
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with Evomela and for 6 months after the last dose.
Males
Evomela administration may damage spermatozoa and testicular tissue, resulting in possible genetic fetal abnormalities. Advise males with female partners of reproductive potential to use effective contraception during treatment with Evomela and for 3 months after the last dose [see Nonclinical Toxicology ( 13.1)].
Infertility
Females
Melphalan causes suppression of ovarian function in premenopausal women, resulting in amenorrhea in a significant number of patients.
Males
Reversible and irreversible testicular suppression has been reported in male patients after administration of melphalan.
8.4 Pediatric Use
Pediatric patients were not included in clinical trials. Safety and effectiveness have not been established in pediatric patients.
8.5 Geriatric Use
Of the total number of subjects in the single-arm pivotal study of Evomela, 30% were 65 and over, but no patients were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. A greater incidence of engraftment syndrome was observed in older patients; 7% (3 of 43) of patients younger than 65 years old versus 28% (5 of 18) of patients 65 years old and over.
10 OVERDOSAGE
Overdoses resulting in death have been reported with melphalan. Overdoses, including doses up to 290 mg/m 2, have produced the following symptoms: severe nausea and vomiting, decreased consciousness, convulsions, muscular paralysis, and cholinomimetic effects. Severe mucositis, stomatitis, colitis, diarrhea, and hemorrhage of the gastrointestinal tract occur at high doses (>100 mg/m 2). Elevations in liver enzymes and veno-occlusive disease occur infrequently. Significant hyponatremia, caused by an associated inappropriate secretion of ADH syndrome, has been observed. Nephrotoxicity and adult respiratory distress syndrome have been reported rarely.
The principal toxic effect is bone marrow suppression leading to leucopenia, thrombocytopenia and anemia. Hematologic parameters should be closely followed for 3 to 6 weeks. An uncontrolled study suggests that administration of autologous bone marrow or hematopoietic growth factors (i.e., sargramostim, filgrastim) may shorten the period of pancytopenia. General supportive measures together with appropriate blood transfusions and antibiotics should be instituted as deemed necessary by the physician. This drug is not removed from plasma to any significant degree by hemodialysis or hemoperfusion. A pediatric patient survived a 254 mg/m 2 overdose treated with standard supportive care.
11 DESCRIPTION
Evomela contains melphalan hydrochloride, an alkylating drug, as the active ingredient. The chemical name of melphalan hydrochloride is 4-[bis(2-chloroethyl)amino]-L-phenylalanine hydrochloride. Its molecular formula is C 13H 18Cl 2N 2O 2 HCl and the molecular weight is 341.67. The structural formula is:
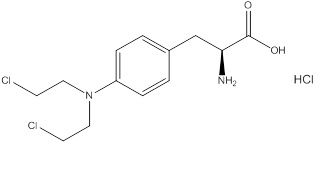
Melphalan hydrochloride is a white to off-white powder, with a melting range of 199°C − 201°C. It is practically insoluble in water, but freely soluble in 1N HCl and methanol.
Evomela (melphalan) for injection is supplied as a sterile white to off-white lyophilized powder in a single-dose vial for intravenous use. Each vial contains 50 mg melphalan free base equivalent to 56 mg melphalan hydrochloride and 2700 mg Betadex Sulfobutyl Ether Sodium, NF.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Melphalan is an alkylating agent of the bischloroethylamine type. As a result, its cytotoxicity appears to be related to the extent of its interstrand cross-linking with DNA, probably by binding at the N 7 position of guanine. Like other bifunctional alkylating agents, it is active against both resting and rapidly dividing tumor cells.
12.2 Pharmacodynamics
The peak mean heart rate increased 20 bpm from baseline following melphalan 100 mg/m 2 for 2 consecutive days in multiple myeloma patients undergoing autologous stem cell transplantation.
Cardiac Electrophysiology
No large mean increase in QTc (i.e. > 20 ms) was detected following melphalan 100 mg/m 2.
12.3 Pharmacokinetics
Mean (± SD) peak plasma concentrations and AUC 0-inf were 5.8 ± 1.5 mcg/mL and 451 ± 109 mcg*min/mL, respectively, following administration of melphalan 100 mg/m 2 in multiple myeloma patients.
Distribution
The volume of distribution of melphalan ranges from approximately 35.5 to 185.7 L/m 2. Melphalan penetrates into cerebrospinal fluid (CSF). Protein binding of melphalan ranges from approximately 50% to 90%, primarily to serum albumin (40% to 60%) and to a lesser extent to α1-acid glycoprotein (20%). Approximately 30% of melphalan is (covalently) irreversibly bound to plasma proteins.
Elimination
Melphalan terminal elimination half-life is approximately 75 minutes. Average total body clearance (CL) ranges from approximately 250 to 325 mL/min/m 2.
Metabolism
Melphalan primarily undergoes chemical hydrolysis to inactive metabolites.
Excretion
Mean values of melphalan excreted in urine range from 5.8% to 21.3%.
Specific Populations
Patient Body Weight
Melphalan clearance changes with ideal body weight (IBW), which decreased by 28% and increased by 31% with IBW of 45 kg and 100 kg compared to 70 kg IBW, respectively.
Renal Impairment
A decrease in estimated creatinine CL from 100 mL/min to 30 mL/min results in 28.2% reduction in CL for a typical person with an IBW of 70 kg.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate and well-controlled carcinogenicity studies have not been conducted in animals. However, intraperitoneal (IP) administration of melphalan in rats (5.4 to 10.8 mg/m 2) and in mice (2.25 to 4.5 mg/m 2) 3 times per week for 6 months followed by 12 months post-dose observation produced peritoneal sarcomas and lung tumors, respectively.
Intramuscular administration of melphalan at 6 and 60 mg/m 2 produced structural aberrations of the chromatid and chromosomes in bone marrow cells of Wistar rats.
14. CLINICAL STUDIES
14.1 Myeloablative Conditioning in Patients with Multiple Myeloma Undergoing ASCT
An open-label, single-arm, non-randomized trial of Evomela was conducted at 5 US centers (NCT 01660633). The 61 patients enrolled had symptomatic multiple myeloma and had at least 2 × 10 6 CD34+ cells/kg cryopreserved stem cells available. The median age was 62 years (range 32 to 73); 57% male, 80% white, 18% black, 2% Asian. Evomela was administered at 100 mg/m 2/day over 30 minutes by IV infusion for two consecutive days (Day -3 and Day -2) prior to ASCT (Day 0).
The objective of the trial was to determine the overall safety and toxicity profile of 200 mg/m 2 of Evomela in patients with multiple myeloma undergoing ASCT. The efficacy was evaluated by the International Myeloma Working Group response criteria comparing the disease response immediately prior to the ASCT procedure to the disease response assessed 90 to 100 days post-transplant. In addition, successful myeloablation, and time to engraftment were evaluated.
The overall response rate (partial response or better) improved from 79% (48 of 61) prior to the ASCT procedure to 95% (58 of 61) at 90 to 100 days post-transplant. There was also an increase in the number of patients with a stringent complete response from 0 patients prior to the ASCT procedure to 16% (10 of 61) at 90 to 100 days post-transplant.
Myeloablation and engraftment were evaluated by complete blood cell count tests daily until neutrophil and platelet engraftment, and then weekly until Day 30, and at Day 60 and Day 90-100. Myeloablation was defined as any of the following: absolute neutrophil count (ANC) < 500/mm 3, absolute lymphocyte count < 100/mm 3, or platelet count < 20,000/mm 3). Neutrophil engraftment was defined as ANC > 500/mm 3 ×3 consecutive daily assessments. Platelet engraftment was defined as untransfused platelet counts > 20,000/mm 3 ×3 consecutive daily assessments. Nonengraftment was defined as failure to reach an ANC > 500/mm 3 ×3 consecutive daily assessments by Day 90-100.
Myeloablation, neutrophil engraftment and platelet engraftment were achieved by all 61 patients. Myeloablation occurred on ASCT Day 5 (range ASCT days -1 to 6) with the median time to myeloablation from dosing of 8 days. The median time to neutrophil engraftment was 12 days (range ASCT days 10 to 16). The median time to platelet engraftment was 13 days (range ASCT days 10 to 28).
15 REFERENCES
- OSHA Hazardous Drugs. OSHA. [Accessed on 9 December 2014, from http://www.osha.gov/SLTC/hazardousdrugs/index.html].
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Evomela is supplied in a single carton containing one (1) vial. Each 50 mg vial contains a white to off- white lyophilized powder in single-dose vial for reconstitution (after reconstitution the solution is clear and coloress to light yellow). Each vial contains 50 mg melphalan free base equivalent to 56 mg melphalan hydrochloride.
NDC: 72893-001-01: Individual carton of Evomela 20 mL single-dose vial containing 50 mg melphalan free base.
Storage and Handling
Store Evomela at room temperature 25°C (77°F). Temperature excursions are permitted between 15- 30°C (59-86°F). [see USP Controlled Room Temperature]
Evomela is light sensitive. Retain in original carton until use.
Melphalan is a hazardous drug. Follow applicable special handling and disposal procedures. 1
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise patients or their caregivers of the following:
Low Blood Cell Counts
- To report any signs or symptoms of thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia. Inform patients of the need for routine blood counts [see Warnings and Precautions ( 5.1)] .
Mucositis
- Inform patients of the signs and symptoms of mucositis. Instruct patients on ways to reduce the risk of its development, and on ways to maintain nutrition and control discomfort if it occurs [see Warnings and Precautions ( 5.2)] .
Nausea, Vomiting and Diarrhea
- To report symptoms of nausea, vomiting and diarrhea, so that appropriate antiemetic and/or antidiarrheal medications can be administered [see Warnings and Precautions ( 5.2)] .
Allergic Reactions
- To immediately report symptoms of hypersensitivity reactions including changes involving the skin, breathing or heart rate, so that antihistamine or corticosteroid therapy can be administered [see Warnings and Precautions ( 5.4)] .
Secondary cancers
- To understand the potential long-term risks related to secondary malignancy [see Warnings and Precautions ( 5.5 )] .
Embryo-Fetal Toxicity
- Advise pregnant women of the potential risk to a fetus [see Warnings and Precautions ( 5.6) and Use in Specific Populations ( 8.1)] .
- Advise females of reproductive potential to use effective contraception during treatment with Evomela and for 6 months after the last dose. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking Evomela [see Warnings and Precautions ( 5.6) and Use in Specific Populations ( 8.1, 8.3)] .
- Inform both females and males of reproductive potential about the risk for infertility [see Warnings and Precautions ( 5.7) and Use in Specific Populations ( 8.3)] .
- Advise males with female partners of reproductive potential to use effective contraception during treatment with Evomela and for 3 months after the last dose [see Use in Specific Populations ( 8.3)] .
Lactation
- Advise women not to breastfeed during treatment with Evomela and for one week after the last dose [see Use in Specific Populations ( 8.2)] .
Manufactured For:
Acrotech Biopharma LLC
279 Princeton Hightstown Rd.
East Windsor, NJ 08520
PATIENT INFORMATION
EVOMELA (ev-o-meh-lah)
(melphalan) for injection, for intravenous use
What is Evomela?
Evomela is a prescription medicine used in people with a type of cancer called multiple myeloma before receiving a stem cell transplant (conditioning treatment).
It is not known if Evomela is safe and effective in children.
Do not receive Evomela if you are allergic to melphalan or any of the ingredients in Evomela. See the end of this leaflet for a complete list of ingredients in Evomela.
Before you receive Evomela, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection
- have had chemotherapy treatment
- have nausea, vomiting, or diarrhea
- have liver or kidney problems
- are pregnant or plan to become pregnant. Evomela can harm your unborn baby.
Females who can become pregnant:
o You should not become pregnant during treatment with Evomela.
o You should use effective birth control (contraception) during treatment and for 6 months after your last dose of Evomela.
o Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Evomela.
Males with female partners who can become pregnant:
o You should use effective birth control (contraception) during treatment and for 3 months after your last dose of Evomela.
- are breastfeeding or plan to breastfeed. It is not known if Evomela passes into your breast milk. You should not breastfeed during treatment with Evomela.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive Evomela?
- Evomela is given to you into your vein through an intravenous (IV) line over 30 minutes.
- Your healthcare provider will do blood tests before and during your treatment with Evomela.
- Your healthcare provider will prescribe medicines to help prevent nausea.
What are the possible side effects of Evomela?
Evomela may cause serious side effects, including:
- Low blood cell counts are common with Evomela and can be serious. Your healthcare provider will do blood tests as needed to check your blood counts during your treatment with Evomela.
ᴼ
Low platelet counts: Tell your healthcare provider right away if you have unusual bleeding or bruising under your skin.
ᴼ
Low red blood cell counts: Tell your healthcare provider if you are feeling weak, tired, or you get tired easily, you look pale, or you feel short of breath.
ᴼ
Low white blood cell counts: A low white blood cell count can cause you to get infections, which may be serious. Tell your healthcare provider right away if you have symptoms of infection, such as fever, chills, cough, pain or burning during urination.
-
Redness and sores of the lining of the mouth, lips, throat, stomach, and genitals (mucositis). Mucositis is common during treatment with Evomela, and can sometimes be severe. Mucositis may cause discomfort or pain.
Your healthcare provider will tell you about ways to maintain nutrition and help control the discomfort from mucositis, and may prescribe medicines if needed. - Nausea, vomiting, and diarrhea are common with Evomela and can sometimes be serious. Tell your healthcare provider if you get nausea, vomiting, or diarrhea. Your healthcare provider may prescribe medicines to help prevent or treat these side effects.
-
Liver problems. Your healthcare provider will check you for liver problems during treatment with Evomela
. Tell your healthcare provider right away if you get any of the following signs or symptoms:
ᴼ yellowing of your skin or the whites of your eyes
ᴼ pain on the right side of your stomach-area (abdomen)
ᴼ severe nausea or vomiting
ᴼ dark urine (tea colored)
-
Serious Allergic reactions. Tell your healthcare provider right away if you get any of the following signs or symptoms:
ᴼ skin reactions, including welts, rash, itching, and redness
ᴼ fast heartbeat
ᴼ shortness of breath or trouble breathing
ᴼ feel lightheaded or dizzy
ᴼ blurry vision
ᴼ swelling of your face, tongue, or thro
ᴼ chest tightness
ᴼ wheezingt
Your healthcare provider may need to discontinue treatment with Evomela if you have a serious allergic reaction.
- Secondary cancers. New cancers have happened in people who have been treated with Evomela.
- Infertility. Evomela may cause fertility problems in males and females. Talk to your healthcare provider if this is a concern for you. In females, your menstrual periods may stop due to treatment with Evomela.
The most common side effects of Evomela include tiredness and low potassium level.
These are not all the possible side effects of Evomela.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Evomela.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about Evomela that is written for health professionals.
What are the ingredients in Evomela?
Active ingredient: melphalan hydrochloride
Inactive ingredient: Betadex Sulfobutyl Ether Sodium
Manufactured for:
Acrotech Biopharma LLC
279 Princeton Hightstown Rd.
East Windsor, NJ 08520
For more information, go to www.evomela.com or call 1-888-292-9617.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: August 2021
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
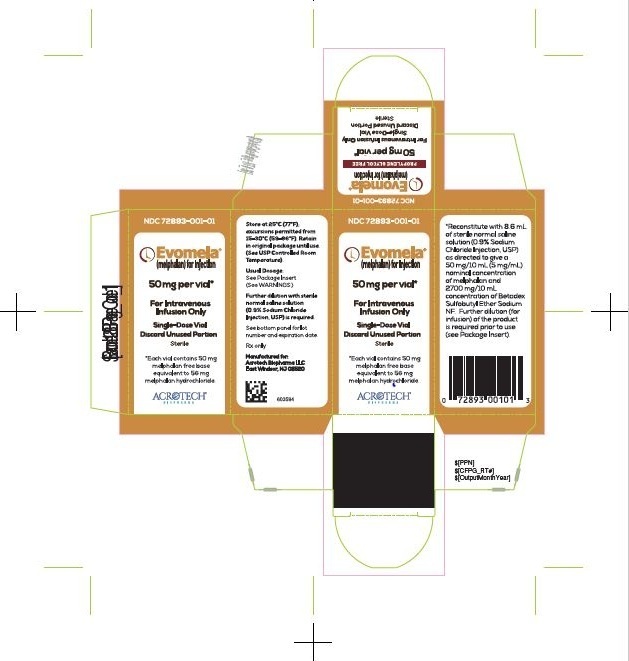
Evomela Carton Label
NDC: 72893-001-01
Evomela ® (melphalan) for Injection
50 mg per vial*
For Intravenous Infusion Only
Single-Use Vial
Discard Unused Portion
Sterile
*Each vial contains 50 mg melphalan free base equivalent to 56 mg melphalan hydrochloride.
Acrotech Biopharma LLC.

Evomela Vial Label
NDC: 72893-001-01
Evomela ® (melphalan) for Injection
50 mg per vial*
For Intravenous Infusion Only
Single-Use Vial Discard Unused Portion
Sterile
*Each vial contains 50 mg melphalan free base equivalent to 56 mg melphalan hydrochloride.
Rx only
| EVOMELA
melphalan injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CENEXI - BLA (370088959) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cenexi-Laboratoires Thissen S.A | 370088959 | manufacture(69605-001) | |
Trademark Results [EVOMELA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EVOMELA 86508057 4994159 Live/Registered |
ACROTECH BIOPHARMA LLC 2015-01-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.