ILLUCCIX- kit for the preparation of gallium ga 68 gozetotide injection kit
ILLUCCIX by
Drug Labeling and Warnings
ILLUCCIX by is a Prescription medication manufactured, distributed, or labeled by Telix Pharmaceuticals (US) Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ILLUCCIX safely and effectively. See full prescribing information for ILLUCCIX.
ILLUCCIX® (kit for the preparation of gallium Ga 68 gozetotide injection), for intravenous use
Initial U.S. Approval: 2020RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ILLUCCIX, after radiolabeling with Ga 68, is a radioactive diagnostic agent indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer:
- With suspected metastasis who are candidates for initial definitive therapy
- With suspected recurrence based on elevated serum prostate-specific antigen (PSA) level
- For selection of patients who are indicated for PSMA-directed therapy as described in the prescribing information of the therapeutic products ( 1)
DOSAGE AND ADMINISTRATION
- Use appropriate aseptic technique and radiation safety handling measures in the manipulation and administration of Gallium Ga 68 Gozetotide Injection. ( 2.1)
- The recommended amount of radioactivity for adults is 111 MBq to 259 MBq (3 mCi to 7 mCi) as a bolus intravenous injection. ( 2.2)
- A diuretic expected to act within the uptake time period may be administered at the time of radiotracer injection. ( 2.2)
- Initiate imaging 50 minutes to 100 minutes after administration. The patient should void immediately prior to initiation of imaging. The scan should begin caudally and proceed cranially. ( 2.6)
- See full prescribing information for additional preparation, administration, imaging, and radiation dosimetry information. ( 2)
DOSAGE FORMS AND STRENGTHS
ILLUCCIX is supplied as a kit containing:
- Vial 1 (Gozetotide Vial) contains 25 mcg gozetotide and 10 mcg D-mannose (stabilizer) as a lyophilized powder
- Vial 2 (Acetate Buffer Vial) contains acetate buffer, either:
- Vial 2A in Configuration A for use with cyclotron produced Ga 68 via GE FASTlab TMor EZAG generator
- Vial 2B in Configuration B for use with IRE generator
- Vial 3 (Sterile Vacuumed Reaction Vial) for the collection of gallium Ga 68 chloride and radiolabeling reaction
After radiolabeling and pH adjustment with acetate buffer and radiolabeling with Ga 68, Vial 3 is a multiple-dose vial containing up to 1,850 MBq (50 mCi) of Gallium Ga 68 Gozetotide Injection in 7.5 mL at a strength of up to 247 MBq (6.7 mCi) per mL. ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
- Risk for Misinterpretation: Gallium Ga 68 gozetotide uptake can be seen in a variety of tumor types and in non-malignant processes. Interpretation of ILLUCCIX PET imaging with histopathology and/or other diagnostic procedures is recommended. ( 5.1)
- Radiation Risks: Ensure safe handling to protect patients and health care providers from unintentional radiation exposure. ( 2.1, 5.2)
ADVERSE REACTIONS
The most commonly reported adverse reactions (incidence ≥ 0.5%) include fatigue, nausea, constipation, and vomiting. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Telix Pharmaceuticals (US) Inc. at 1-844-455-8638 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
2.2 Recommended Dosage and Administration Instructions
2.3 Patient Preparation Prior to PET Imaging
2.4 Drug Preparation
2.5 Specifications and Quality Control
2.6 Image Acquisition
2.7 Image Interpretation
2.8 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Misinterpretation
5.2 Radiation Risks
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Imaging Prior to Initial Definitive Therapy
14.2 Imaging of Biochemical Recurrence
14.3 Imaging to Select Patients for PSMA-Directed Therapy
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ILLUCCIX, after radiolabeling with Ga 68, is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer:
- With suspected metastasis who are candidates for initial definitive therapy
- With suspected recurrence based on elevated serum prostate-specific antigen (PSA) level
- For selection of patients who are indicated for PSMA-directed therapy as described in the prescribing information of the therapeutic products
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
After reconstitution and radiolabeling of ILLUCCIX, the vial contains Gallium Ga 68 Gozetotide Injection. Handle Gallium Ga 68 Gozetotide Injection with appropriate safety measures to minimize radiation exposure [ see Warnings and Precautions (5.2)]. Use waterproof gloves, effective radiation shielding, and other appropriate safety measures when preparing and handling Gallium Ga 68 Gozetotide Injection.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Recommended Dosage and Administration Instructions
Recommended Dosage
In adults, the recommended amount of radioactivity to be administered for PET is 111 MBq to 259 MBq (3 mCi to 7 mCi) administered as an intravenous bolus injection.
Administration
- Use aseptic technique and radiation shielding when withdrawing and administering Gallium Ga 68 Gozetotide Injection.
- Calculate the necessary volume to administer based on calibration time and required dose.
- Inspect Gallium Ga 68 Gozetotide Injection visually for particulate matter and discoloration before administration. Only use solutions that are clear, colorless or at most slightly yellow, and without visible particles.
- Gallium Ga 68 Gozetotide Injection may be diluted with sterile 0.9% Sodium Chloride Injection, USP.
- Assay the final dose in a dose calibrator immediately before administration to the patient.
- After injection of Gallium Ga 68 Gozetotide Injection, administer an intravenous flush of sterile 0.9% Sodium Chloride Injection, USP to ensure full delivery of the dose.
- Dispose of any unused drug in a safe manner in compliance with applicable regulations.
- Unless contraindicated, a diuretic expected to act within the uptake time period may be administered at the time of radiotracer injection to potentially decrease artifact from radiotracer accumulation in the urinary bladder and ureters.
2.3 Patient Preparation Prior to PET Imaging
Instruct patients to drink a sufficient amount of water to ensure adequate hydration prior to administration of Gallium Ga 68 Gozetotide Injection and to continue to drink and void frequently following administration to reduce radiation exposure, particularly during the first hour after administration [see Warnings and Precautions (5.2)].
2.4 Drug Preparation
ILLUCCIX is supplied as a 3-vial kit in two different configurations [see Dosage Forms and Strengths (3)] for preparation of Gallium Ga 68 Gozetotide Injection with eluate from one of the following (see below for specific instructions for use with each Ga 68 source):
- Cyclotron produced via GE FASTlab TM(Configuration A)
- Eckert & Ziegler (EZAG) GalliaPharm ®Germanium 68/Gallium 68 (Ge 68/Ga 68) generator (Configuration A)
- IRE Galli Eo ®Ge 68/Ga 68 generator (Configuration B)
The Ge 68/Ga 68 generators and cyclotron are not supplied with ILLUCCIX.
Components of ILLUCCIX include:
- Vial 1 (Gozetotide Vial) contains 25 mcg of gozetotide and 10 mcg of D-mannose (stabilizer) as a lyophilized powder.
- Vial 2 (Acetate Buffer Vial) contains 150 mg anhydrous sodium acetate in HCl buffer.
- Vial 3 (Sterile Vacuumed Reaction Vial) is a sterile, evacuated vial that serves as the collection vial for Ga 68 chloride and radiolabeling reaction.
Prepare Gallium Ga 68 Gozetotide Injection according to the following aseptic procedure:
- Before use, remove the ILLUCCIX kit containing all three components from the refrigerator and allow the kit to reach room temperature for 1 hour.
- Use suitable shielding to reduce radiation exposure.
- Wear waterproof gloves.
- If Ga 68 is generator produced, test the Ga 68 chloride eluate for Ge 68 breakthrough weekly by a suitable method according to manufacturer recommendations. Ge 68 breakthrough and other gamma emitting radionuclides should meet the specifications (≤0.001%) provided by the manufacturer. If Ga 68 is cyclotron produced, test for Ga 66 and Ga 67 (with specification of ≤2% combined total) when a new lot of Zn 68 is introduced for manufacturing.
- Place a "radioactive" label on Vial 3 (Sterile Vacuumed Reaction Vial) with product name, lot number and date.
- Remove the vial cap from Vial 1, Vial 2 and Vial 3.
- Swab the top of each vial with alcohol to disinfect the surface and allow the top of each vial to dry.
- Note that to minimize any potential metallic contamination, the shortest possible needle should be used for the transfer of the gallium solution from the generator. The needle should be clean and dilute acid resistant.
- Use only plastic syringes for preparation and administration. Do notuse syringes with rubber plungers.
- Note that prior to use of any vial, confirm the correct vial is being used by a visual check of the vial label.
- Follow the specific reconstitution procedure below, dependent on Ga 68 source. Then continue with the dilution and radiosynthesis procedure below.
Preparation with Cyclotron Produced Ga 68 via GE FASTlab
Collection of Gallium Ga 68 Chloride
- After purification by the FASTlab, the Ga 68 chloride solution is passed through a sterile filter and into the cassette product vial automatically by the FASTlab.
- Pierce Vial 3 (Sterile Vacuumed Reaction Vial) with a sterile needle connected to a 0.2 micron sterile vented filter (not supplied) to maintain atmospheric pressure within the vial during the reconstitution process.
- Aseptically transfer 5 mL of Ga 68 chloride solution into Vial 3.
Reconstitution and Radiolabeling Reaction Procedure
- Insert a sterile 10 mL syringe with a needle into Vial 2 (Acetate Buffer Vial Configuration A) and draw up the contents of the vial (target 2.5 mL).
- Inject the contents of the 10 mL syringe into Vial 1 (Gozetotide Vial).
- Gently swirl Vial 1 to ensure the product is thoroughly dissolved.
- Insert a sterile 10 mL syringe with a needle into Vial 1 containing the dissolved gozetotide and draw up the entire contents of the vial.
- Transfer the contents of the 10 mL syringe to Vial 3 (Sterile Vacuumed Reaction Vial) containing the Ga 68 chloride.
- Wait for 5 minutes for radiolabeling to take place at ambient temperature (25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)).
- Assay the whole vial containing the Gallium Ga 68 Gozetotide Injection for total radioactivity using a dose calibrator, calculate the radioactivity concentration and record the result.
- Perform the quality control of Gallium Ga 68 Gozetotide Injection according to the recommended methods in the Subsection 2.5Specifications and Quality Control.
- Prior to use, visually inspect the solution behind a shielded screen for radioprotection purposes. Only use solutions that are clear without visible particles.
- Keep the vial containing the Gallium Ga 68 Gozetotide Injection upright in a radio-protective shield container at ambient temperature until use.
- After reconstitution and addition of Ga 68 chloride to the kit components in the reaction Vial 3, use Gallium Ga 68 Gozetotide Injection within 4 hours. The final volume of the Gallium Ga 68 Gozetotide Injection is 7.5 mL.
Preparation with Eckert & Ziegler GalliaPharm Generator
Collection of Gallium Ga 68 Chloride
- Prepare a syringe containing 5 mL of sterile ultrapure 0.1 M HCl provided with the GalliaPharm Generator for elution.
- Pierce Vial 3 (Sterile Vacuumed Reaction Vial) with a sterile needle connected to a 0.2 micron sterile vented filter (not supplied) to maintain atmospheric pressure within the vial during the reconstitution process.
- Connect the male luer of the outlet line of the GalliaPharm generator to a sterile needle.
- Connect Vial 3 directly to the outlet line of the GalliaPharm generator by pushing the needle through the rubber septum and place the vial in a radiation shielded container.
- Elute the generator directly with the 5 mL 0.1 M HCl from step 1 into Vial 3 according to the instructions for use of the GalliaPharm generator that are supplied by Eckert & Ziegler. Perform the elution manually or by means of a pump. Collect 5 mL of eluate.
- At the end of the elution, disconnect the generator from Vial 3 by removing the needle from the rubber septum.
Reconstitution and Radiolabeling Reaction Procedure
- Insert a sterile 10 mL syringe with a needle into Vial 2 (Acetate Buffer Vial Configuration A) and draw up the contents of the vial (target 2.5 mL).
- Inject the contents of the 10 mL syringe into Vial 1 (Gozetotide Vial).
- Gently swirl Vial 1 to ensure the product is thoroughly dissolved.
- Insert a sterile 10 mL syringe with a needle into Vial 1 containing the dissolved gozetotide and draw up the entire contents of the vial.
- Transfer the contents of the 10 mL syringe to Vial 3 (Sterile Vacuumed Reaction Vial) containing the Ga 68 chloride.
- Wait for 5 minutes for radiolabeling to take place at ambient temperature (25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)).
- Assay the whole vial containing the Gallium Ga 68 Gozetotide Injection for total radioactivity using a dose calibrator, calculate the radioactivity concentration and record the result.
- Perform the quality control of Gallium Ga 68 Gozetotide Injection according to the recommended methods in the Subsection 2.5Specifications and Quality Control.
- Prior to use, visually inspect the solution behind a shielded screen for radioprotection purposes. Only use solutions that are clear without visible particles.
- Keep the vial containing the Gallium Ga 68 Gozetotide Injection upright in a radio-protective shield container at ambient temperature until use.
- After reconstitution and addition of Ga 68 chloride to the kit components in the reaction Vial 3, use Gallium Ga 68 Gozetotide Injection within 4 hours. The final volume of the Gallium Ga 68 Gozetotide Injection is 7.5 mL.
Preparation with IRE Galli Eo Generator
Collection of Gallium Ga 68 Chloride
- Connect the male luer of the outlet line of the Galli Eo generator to a sterile needle.
- Elute the generator directly into Vial 3 (Sterile Vacuumed Reaction Vial) according to the instructions for use of the Galli Eo generator that are supplied by IRE. Collect 1.1 mL of eluate.
- At the end of the elution, disconnect the generator from Vial 3 by removing the needle from the rubber septum.
Reconstitution and Radiolabeling Reaction Procedure
- Insert a sterile 10 mL syringe with a needle into Vial 2 (Acetate Buffer Vial Configuration B) and draw up the contents of the vial (target 6.4 mL).
- Inject the contents of the 10 mL syringe into Vial 1 (Gozetotide Vial).
- Gently swirl Vial 1 to ensure the product is thoroughly dissolved.
- Insert a sterile 10 mL syringe with a needle into Vial 1 containing the dissolved gozetotide and draw up the entire contents of the vial.
- Transfer the contents of the 10 mL syringe to Vial 3 (Sterile Vacuumed Reaction Vial) containing the Ga 68 chloride.
- Wait for 5 minutes for radiolabeling to take place at ambient temperature (25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)).
- Assay the whole vial containing the Gallium Ga 68 Gozetotide Injection for total radioactivity using a dose calibrator, calculate the radioactivity concentration and record the result.
- Perform the quality control of Gallium Ga 68 Gozetotide Injection according to the recommended methods in the Subsection 2.5Specifications and Quality Control.
- Prior to use, visually inspect the solution behind a shielded screen for radioprotection purposes. Only use solutions that are clear without visible particles.
- Keep the vial containing the Gallium Ga 68 Gozetotide Injection upright in a radio-protective shield container at ambient temperature until use.
- After reconstitution and addition of Ga 68 chloride to the kit components in the reaction Vial 3, use Gallium Ga 68 Gozetotide Injection within 4 hours. The final volume of the Gallium Ga 68 Gozetotide Injection is 7.5 mL.
Flow diagrams are provided for the radiosynthetic process to be followed at the radiopharmacy site for:
- ILLUCCIX Configuration "A" when using Ga 68 prepared by cyclotron ( Figure 1) or
- ILLUCCIX Configurations "A" or "B" when using an indicated GMP-grade EZAG GalliaPharm Ge 68/Ga 68 Generator ( Figure 2) or IRE Galli Eo Ge 68/Ga 68 Generator ( Figure 3).
Figure 1: Preparation with Cyclotron Produced Ga 68 via GE FASTlab
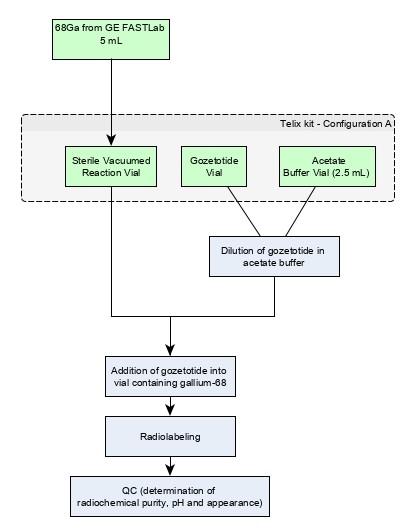
Note: Ga 68 from GE FASTlab is aseptically transferred into the Sterile Vacuumed Reaction Vial.
Use "Configuration A" with cyclotron produced Ga 68 via GE FASTlab [see How Supplied/Storage and Handling (16)].
Figure 2: Preparation with Eckert & Ziegler GalliaPharm Generator
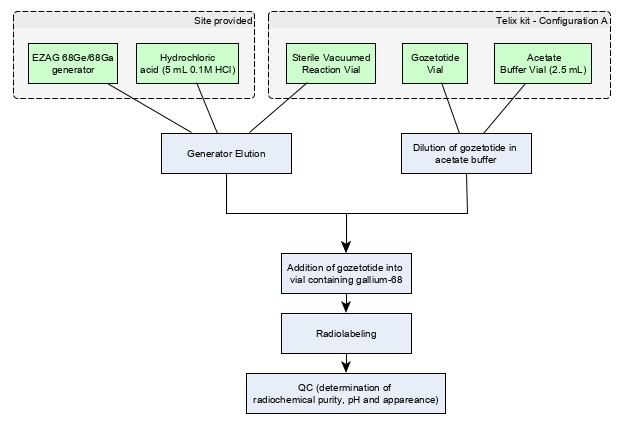
Note: Use "Configuration A" with EZAG GalliaPharm Ge 68/Ga 68 generator [see How Supplied/Storage and Handling (16)].
Figure 3: Preparation with IRE Galli Eo Generator
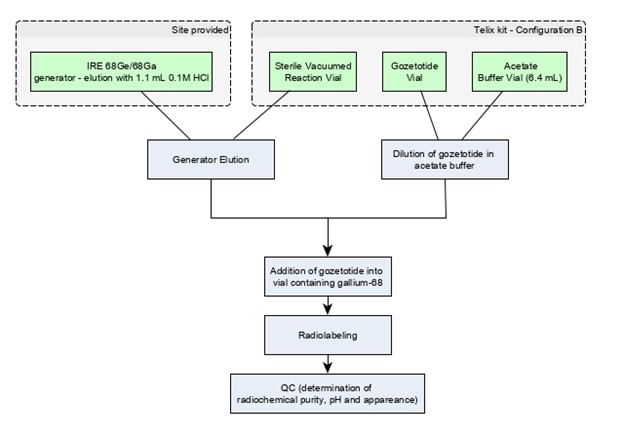
Note: Use "Configuration B" with IRE Galli Eo Ge 68/Ga 68 generator [see How Supplied/Storage and Handling (16)].
2.5 Specifications and Quality Control
Perform the quality controls in Table 1behind a lead glass shield for radioprotection purposes.
Table 1: Specifications for the Radiolabeled Imaging Product (Gallium Ga 68 Gozetotide Injection)
Test
Analytical method
Acceptance criteria
Appearance
Visual examination
Colorless to slightly yellow solution
Free from visible particles
pH
pH-meter or pH-strips
4.0 to 5.0
Radiochemical purity
- Content of gallium Ga 68 gozetotide
- Content of free and colloidal Ga 68
Instant thin-layer chromatography, silica gel (iTLC SG);
See methods below≥95%
≤5%Perform one of the following:
Cutting Technique
- Pour ammonium acetate 1 M/methanol (1/1 V/V) solution to a depth of 3 mm to 4 mm in the developing chamber, cover the chamber and allow it to equilibrate.
- Draw a pencil line at 1 cm from the bottom of the iTLC strip, and a dotted line 5 cm from the pencil line. Apply a drop of gallium Ga 68 gozetotide at the center of the pencil line.
- Place the iTLC strip in the developing chamber and allow it to develop for a distance of 10 cm from the point of application.
- Cut the iTLC strip following the dotted line and measure each piece with the radioactivity dose calibrator.
- Calculate the quantity (in percent) of gallium Ga 68 gozetotide in the solution using the formula:
% Gallium Ga 68 gozetotide=(Activity top piece)/(Activity bottom piece+Activity top piece) ×100
Scanning Technique
- Pour ammonium acetate 1 M/methanol (1/1 V/V) solution to a depth of 3 mm to 4 mm in the developing chamber, cover the chamber and allow it to equilibrate.
- Draw a pencil line at 1 cm from the bottom of the iTLC strip and apply a drop of gallium Ga 68 gozetotide at the center of the pencil line.
- Place the iTLC strip in the developing chamber and allow it to develop for a distance of 10 cm from the point of application.
- Scan the iTLC with a radiometric iTLC scanner.
- Calculate the quantity (in percent) of gallium Ga 68 gozetotide in the solution by integration of the peaks on the chromatogram.
- The retention factor (Rf) specifications are:
Free and colloidal Ga 68 species, Rf = 0 to 0.1,
Gallium Ga 68 gozetotide, Rf = 0.6 to 1.
2.6 Image Acquisition
Position the patient supine with arms above the head. Begin PET scanning 50 minutes to 100 minutes after the intravenous administration of Gallium Ga 68 Gozetotide Injection. Patients should void immediately prior to image acquisition and image acquisition should begin at the proximal thighs and proceed cranially to the skull base or skull vertex. Adapt imaging technique according to the equipment used and patient characteristics in order to obtain the best image quality possible.
2.7 Image Interpretation
Gallium Ga 68 gozetotide binds to PSMA. Based on the intensity of the signals, PET images obtained using gallium Ga 68 gozetotide indicate the presence of PSMA in tissues.
Imaging Prior to Initial Definitive Therapy or for Biochemical Recurrence
Lesions should be considered suspicious if uptake is greater than physiologic uptake in that tissue or greater than adjacent background if no physiologic uptake is expected. Tumors that do not express PSMA will not be visualized. Increased uptake in tumors is not specific for prostate cancer [ see Warnings and Precautions ( 5.1) ].
Imaging to Select Patients for PSMA-Directed Therapy
For instructions on patient selection, refer to the prescribing information of the PSMA-directed therapeutic product.
2.8 Radiation Dosimetry
Estimated radiation absorbed doses per injected activity for organs and tissues of adult male patients following an intravenous bolus of Gallium Ga 68 Gozetotide Injection are shown in Table 2.
The effective radiation dose resulting from the administration of 259 MBq (7 mCi) is about 4.4 mSv. The radiation doses for this administered dose to the critical organs, which are the kidneys, urinary bladder, and spleen, are 96.2 mGy, 25.4 mGy, and 16.8 mGy, respectively.
These radiation doses are for Gallium Ga 68 Gozetotide Injection alone. If CT or a transmission source are used for attenuation correction, the radiation dose will increase by an amount that varies by technique.
Table 2: Estimated Radiation Absorbed Dose per Injected Activity in Selected Organs and Tissues of Adults after Intravenous Administration of Gallium Ga 68 Gozetotide Injection
Organ
Absorbed dose (mGy/MBq)
Mean
SD
Adrenals
0.0156
0.0014
Brain
0.0104
0.0011
Breasts
0.0103
0.0011
Gallbladder
0.0157
0.0012
Lower Colon
0.0134
0.0009
Small Intestine
0.014
0.002
Stomach
0.0129
0.0008
Heart
0.012
0.0009
Kidneys
0.3714
0.0922
Liver
0.0409
0.0076
Lungs
0.0111
0.0007
Muscle
0.0103
0.0003
Pancreas
0.0147
0.0009
Red Marrow
0.0114
0.0016
Skin
0.0091
0.0003
Spleen
0.065
0.018
Testes
0.0111
0.0006
Thymus
0.0105
0.0006
Thyroid
0.0104
0.0006
Urinary Bladder
0.0982
0.0286
Total Body
0.0143
0.0013
Effective Dose (mSv/MBq)
0.0169
0.0015
-
3 DOSAGE FORMS AND STRENGTHS
ILLUCCIX is supplied as a kit for the preparation of Gallium Ga 68 Gozetotide Injection. Each kit contains:
- Vial 1 (Gozetotide Vial): 25 mcg of gozetotide and 10 mcg of D-mannose (stabilizer) as a lyophilized powder.
- Vial 2 (Acetate Buffer Vial): 150 mg anhydrous sodium acetate in HCl buffer, provided as either vial 2A or vial 2B, depending on Ga 68 source used.
- Vial 3 (Sterile Vacuumed Reaction Vial): a sterile, evacuated vial that serves as the collection vial for Ga 68 chloride and radiolabeling reaction.
Ga 68 is obtained from one of the following sources:
- Cyclotron via GE FASTlab.
- Eckert & Ziegler GalliaPharm Ge 68/Ga 68 generator
- IRE Galli Eo Ge 68/Ga 68 generator.
After reconstitution and pH adjustment with acetate buffer and radiolabeling with Ga 68, Vial 3 is a multiple-dose vial containing up to 1,850 MBq (50 mCi) of Gallium Ga 68 Gozetotide Injection in 7.5 mL at a strength of up to 247 MBq (6.7 mCi) per mL.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Misinterpretation
Image interpretation errors can occur with ILLUCCIX PET. A negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. Gallium Ga 68 gozetotide uptake is not specific for prostate cancer and may occur with other types of cancer as well as non-malignant processes such as Paget's disease, fibrous dysplasia, and osteophytosis. Clinical correlation, which may include histopathological evaluation of the suspected prostate cancer site, is recommended.
The performance of ILLUCCIX for imaging of biochemically recurrent prostate cancer seems to be affected by serum PSA levels and by site of disease. The performance of ILLUCCIX for imaging of metastatic pelvic lymph nodes prior to initial definitive therapy seems to be affected by Gleason score [see Clinical Studies (14.1, 14.2)] .
5.2 Radiation Risks
Gallium Ga 68 gozetotide contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Ensure safe handling to minimize radiation exposure to the patient and health care providers. Advise patients to hydrate before and after administration and to void frequently after administration [see Dosage and Administration (2.1,2.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ILLUCCIX has been established based on three prospective studies of gallium Ga 68 gozetotide in patients with prostate cancer (i.e., Studies 1, 2, and 3). Adverse reactions from these studies are reported below.
In Studies 1 and 2 using another formulation of gallium Ga 68 gozetotide injection, 960 patients received one dose of gallium Ga 68 gozetotide intravenously with the amount (mean ± SD) of radioactivity 188.7 ± 40.7 MBq (5.1 ± 1.1 mCi) [ see Clinical Studies ( 14.1, 14.2) ]. The most commonly reported adverse reactions were nausea, diarrhea, and dizziness, occurring at a rate of <1%.
In a randomized, multicenter, open-label clinical study (NCT03511664, referred to as Study 3) in which gallium Ga 68 gozetotide was used to identify PSMA-positive patients on PET imaging to determine eligibility for PSMA-directed therapy, 1,003 patients with progressive metastatic castration-resistant prostate cancer (mCRPC) received one dose of gallium Ga 68 gozetotide intravenously with the amount of radioactivity 167.1 ± 23.1 MBq (4.52 ± 0.62 mCi). Patients were males with median age of 70 years (range, 40 to 94 years), were White (87%), Black or African American (7%), or Asian (2.4%), and had median baseline PSA levels of 74 ng/mL (range, 0 to 8,995 ng/mL).
Adverse reactions occurring at ≥ 0.5% in patients with metastatic prostate cancer who received gallium Ga 68 gozetotide injection in Study 3 are presented in Table 3.
Table 3: Adverse Reactions (≥ 0.5%) in Patients with Metastatic Prostate Cancer Who Received Gallium Ga 68 Gozetotide Injection in Study 3
Adverse Reaction
Gallium Ga 68 Gozetotide Injection
N = 1,003
n (%)
General disorders
Fatigue
12 (1.2)
Gastrointestinal disorders
Nausea
8 (0.8)
Constipation
5 (0.5)
Vomiting
5 (0.5)
Adverse reactions occurring at a rate of < 0.5% in the study were diarrhea, dry mouth, injection site reactions, including injection site hematoma and injection site warmth and chills.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ILLUCCIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
Androgen deprivation therapy and other therapies targeting the androgen pathway
Androgen deprivation therapy (ADT) and other therapies targeting the androgen pathway, such as androgen receptor antagonists, can result in changes in uptake of gallium Ga 68 gozetotide in prostate cancer. The effect of these therapies on performance of gallium Ga 68 gozetotide PET has not been established.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
ILLUCCIX is not indicated for use in females. There are no available data with gallium Ga 68 gozetotide injection use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. All radiopharmaceuticals, including ILLUCCIX, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. Animal reproduction studies have not been conducted with gallium Ga 68 gozetotide.
8.2 Lactation
Risk Summary
ILLUCCIX is not indicated for use in females. There are no data on the presence of gallium Ga 68 gozetotide in human milk, the effect on the breastfed infant, or the effect on milk production.
8.4 Pediatric Use
The safety and effectiveness of gallium Ga 68 gozetotide in pediatric patients have not been established.
8.5 Geriatric Use
The efficacy of gallium Ga 68 gozetotide PET in geriatric patients with prostate cancer is based on data from three prospective studies.
Of the total number of patients in Studies 1 and 2 [see Clinical Studies (14.1, 14.2)] , 691 of 960 (72%) patients were 65 years of age and older while 195 (20%) were 75 years of age and older.
Of the total number of patients in Study 3 [ see Adverse Reactions (6.1)], 752 of 1,003 (75%) patients were 65 years of age and older while 284 (28%) were 75 years of age and older.
The efficacy and safety profiles of gallium Ga 68 gozetotide appear similar in younger adult and geriatric patients with prostate cancer and other reported clinical experience has not identified differences in responses between the elderly and younger adult patients.
-
10 OVERDOSAGE
In the event of an overdose of gallium Ga 68 gozetotide, reduce the radiation absorbed dose to the patient where possible by increasing the elimination of the drug from the body using hydration and frequent bladder voiding. A diuretic might also be considered. If possible, an estimate of the radiation effective dose given to the patient should be made.
-
11 DESCRIPTION
11.1 Chemical Characteristics
ILLUCCIX, a radioactive diagnostic agent, is supplied as a sterile, multiple-dose kit for the preparation of Gallium Ga 68 Gozetotide Injection for intravenous use. Gozetotide is also known as PSMA-11.
Gallium Ga 68 gozetotide is a radioconjugate composed of a human prostate specific membrane antigen (PSMA)-targeting ligand peptide conjugated via the acyclic radiometal chelator, N,N'-bis [2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine-N,N'-diacetic acid (HBED-CC) to the radioisotope Ga 68. The amino acid sequence of the gozetotide peptide is Glu-NH-CO-NH-Lys(Ahx), (Ahx = 6-aminohexanoic acid). Gallium Ga 68 gozetotide has a molecular weight of 1011.9 g/mol and its chemical structure is shown in Figure 4.
Figure 4: Chemical Structure of Gallium Ga 68 Gozetotide
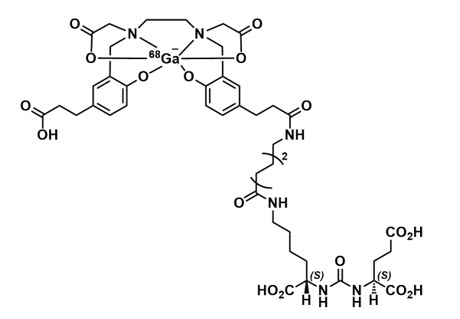
11.2 Physical Characteristics
ILLUCCIX is supplied as a 3-vial kit which contains the non-radioactive ingredients needed to produce Gallium Ga 68 Gozetotide Injection. There are two configurations available to allow preparation of Gallium Ga 68 Gozetotide Injection using Ga 68 from different generator or cyclotron sources [ see How Supplied/Storage and Handling ( 16) ]. The prepared Gallium Ga 68 Gozetotide Injection for intravenous use is a sterile, pyrogen free, clear, colorless, buffered solution with a pH between 4.0 to 5.0.
11.3 External Radiation
Table 4, Table 5, and Table 6display the principal radiation emission data, radiation attenuation by lead shielding, and physical decay of Ga 68.
Table 4: Principal Radiation Emission Data (>1%)
Radiation/ Emission
% Disintegration
Mean Energy (MeV)
beta+
88%
0.8360
beta+
1.1%
0.3526
gamma
178%
0.5110
gamma
3.0%
1.0770
X-ray
2.8%
0.0086
X-ray
1.4%
0.0086
Table 5: Radiation Attenuation of 511 keV Photons by Lead (Pb) Shielding
Shield Thickness (Pb) mm
Coefficient of Attenuation
6
0.5
12
0.25
17
0.1
34
0.01
51
0.001
Table 6: Physical Decay Chart for Ga 68
Minutes
Fraction Remaining
0
1
15
0.858
30
0.736
60
0.541
90
0.398
120
0.293
180
0.158
360
0.025
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gallium Ga 68 gozetotide binds to PSMA. It binds to cells that express PSMA, including malignant prostate cancer cells, which usually overexpress PSMA. Gallium 68 (Ga 68) is a β+ emitting radionuclide that allows positron emission tomography.
12.2 Pharmacodynamics
The relationship between gallium Ga 68 gozetotide plasma concentrations and successful imaging was not explored in clinical trials.
12.3 Pharmacokinetics
Distribution
Intravenously injected gallium Ga 68 gozetotide is cleared from the blood and is accumulated preferentially in the liver (15%), kidneys (7%), spleen (2%), and salivary glands (0.5%). Gallium Ga 68 gozetotide uptake is also seen in the adrenals and prostate. There is no uptake in the cerebral cortex or in the heart, and usually lung uptake is low.
Elimination
A total of 14% of the injected dose is excreted in urine in the first 2 hours post-injection.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Imaging Prior to Initial Definitive Therapy
The efficacy of ILLUCCIX for PET of PSMA-positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy has been established based on a study of another formulation of gallium Ga 68 gozetotide. Below is a display of the results of the prospective, open label study PSMA-PreRP (NCT03368547 and NCT02919111, referred to as Study 1).
This two-center study enrolled 325 patients with biopsy-proven prostate cancer who were considered candidates for prostatectomy and pelvic lymph node dissection. All enrolled patients met at least one of the following criteria: serum prostate-specific antigen (PSA) of at least 10 ng/mL, tumor stage cT2b or greater, or Gleason score greater than 6. Each patient received a single gallium Ga 68 gozetotide PET/CT or PET/MR from mid-thigh to skull base.
A total of 123 patients (38%) proceeded to standard-of-care prostatectomy and template pelvic lymph node dissection and had sufficient histopathology data for evaluation (evaluable patients). Three members of a pool of six central readers independently interpreted each PET scan for the presence of abnormal gallium Ga 68 gozetotide uptake in pelvic lymph nodes located in the common iliac, external iliac, internal iliac, and obturator subregions bilaterally as well as in any other pelvic location. The readers were blinded to all clinical information except for the history of prostate cancer prior to definitive treatment. Extrapelvic sites and the prostate gland itself were not analyzed in this study. For each patient, gallium Ga 68 gozetotide PET results and reference standard histopathology obtained from dissected pelvic lymph nodes were compared by region (left hemipelvis, right hemipelvis, and other).
For the 123 evaluable patients, the mean age was 65 years (range 45 to 76 years), and 89% were White. The median serum PSA was 11.8 ng/mL. The summed Gleason score was 7 for 44%, 8 for 20%, and 9 for 31% of the patients, with the remainder of the patients having Gleason scores of 6 or 10.
Table 7compares majority PET reads to pelvic lymph node histopathology results at the patient-level with region matching, such that at least one true positive region defines a true positive patient. As shown, approximately 24% of subjects studied were found to have pelvic nodal metastases based on histopathology (95% confidence interval: 17%, 32%).
Table 7: Patient-Level Performance of Gallium Ga 68 Gozetotide PET for Detection of Pelvic Lymph Node Metastasis* in Men with Prostate Cancer with Suspected Metastasis in Study 1 (n=123)
Histopathology
Predictive value**
(95% CI)
Positive
Negative
PET scan
Positive
14
9
PPV
61% (41%, 81%)
Negative
16
84
NPV
84% (79%, 91%)
Total
30
93
Diagnostic performance (95% CI)
Sensitivity
47% (29%, 65%)
Specificity
90% (84%, 96%)
*with region matching where at least one true positive region defines a true positive patient
**PPV: positive predictive value, NPV: negative predictive value
Among the pool of six readers, sensitivity ranged from 36% to 60%, specificity from 83% to 96%, positive predictive value from 38% to 80%, and negative predictive value from 80% to 88%.
In an exploratory subgroup analysis based on summed Gleason score, there was a numerical trend toward more true positives in patients with Gleason score of 8 or higher compared to those with Gleason score of 7 or lower.
An exploratory analysis was performed to estimate the sensitivity and specificity for pelvic nodal metastasis detection in all scanned patients, including the patients who were lacking histopathology reference standard. An imputation method was used based on patient-specific factors. This exploratory analysis resulted in an imputed sensitivity of 47%, with a 95% confidence interval ranging from 38% to 55%, and an imputed specificity of 74%, with a 95% confidence interval ranging from 68% to 80% for all patients imaged with gallium Ga 68 gozetotide PET.
14.2 Imaging of Biochemical Recurrence
The efficacy of ILLUCCIX for PET of PSMA-positive lesions in men with prostate cancer with suspected recurrence based on elevated serum PSA level has been established based on a study of another formulation of gallium Ga 68 gozetotide. Below is a display of the results of the prospective, open label study PSMA-BCR (NCT02940262 and NCT02918357, referred to as Study 2).
This two-center study enrolled 635 patients with biochemical evidence of recurrent prostate cancer after definitive therapy, defined by serum PSA of >0.2 ng/mL more than 6 weeks after prostatectomy or by an increase in serum PSA of at least 2 ng/mL above nadir after definitive radiotherapy. All patients received a single gallium Ga 68 gozetotide PET/CT or PET/MR from mid-thigh to skull base. Three members of a pool of nine independent central readers evaluated each scan for the presence and regional location (20 subregions grouped into four regions) of abnormal gallium Ga 68 gozetotide uptake suggestive of recurrent prostate cancer. The readers were blinded to all clinical information other than type of primary therapy and most recent serum PSA level.
A total of 469 patients (74%) had at least one positive region detected by gallium Ga 68 gozetotide PET majority read. The distribution of gallium Ga 68 gozetotide PET positive regions was 34% bone, 25% prostate bed, 25% pelvic lymph node, and 17% extrapelvic soft tissue. Two hundred and ten patients had composite reference standard information collected in a PET positive region (evaluable patients), consisting of at least one of the following: histopathology, imaging (bone scintigraphy, CT, or MRI) acquired at baseline or within 12 months after gallium Ga 68 gozetotide PET, or serial serum PSA. Composite reference standard information for gallium Ga 68 gozetotide PET negative regions was not systematically collected in this study.
In the 210 evaluable patients, the mean age was 70 years (range 49 to 88 years) and 82% were 65 years of age or older. White patients made up 90% of the group. The median serum PSA was 3.6 ng/mL. Prior treatment included radical prostatectomy in 64% and radiotherapy in 73%.
Of the 210 evaluable patients, 192 patients (91%) were found to be true positive in one or more regions against the composite reference standard (95% confidence interval: 88%, 95%). Among the pool of nine readers used in the study, the proportion of patients who were true positive in one or more regions ranged from 82% to 97%. The prostate bed had the lowest proportion of true positive results at the region-level (76% versus 96% for non-prostate regions).
An exploratory analysis was also performed in which gallium Ga 68 gozetotide PET positive patients who lacked reference standard information were imputed using an estimated likelihood that at least one location-matched PET positive lesion was reference standard positive based on patient-specific factors. In this exploratory analysis, 340 of 475 patients (72%) were imputed as true positive in one or more regions (95% confidence interval: 68%, 76%).
In another exploratory analysis using the same imputation approach for PET positive patients who lacked reference standard information, 340 of 635 patients (54%) were correctly detected as true positive (95% confidence interval: 50%, 57%) among all BCR patients who received a PET scan, whether it was read as positive or negative.
The likelihood of identifying a gallium Ga 68 gozetotide PET positive lesion in this study increased with higher serum PSA level. Table 8shows the patient-level gallium Ga 68 gozetotide PET results stratified by serum PSA level. The mean time between PSA measurement and PET scan was 40 days with a range of 0 to 367 days. Percent PET positivity was calculated as the proportion of patients with a positive gallium Ga 68 gozetotide PET out of all patients scanned. Percent PET positivity includes patients determined to be either true positive or false positive as well as those in whom such determination was not made due to the absence of composite reference standard data.
Table 8: Patient-Level Gallium Ga 68 Gozetotide PET Results and Percent PET Positivity Stratified by Serum PSA Level in Men with Prostate Cancer with Suspected Recurrence in Study 2 (n=628)*
PSA (ng/mL)
PET positive patients
PET negative patients
Percent PET positivity***
(95% CI)
Total
TP**
FP**
Without reference standard
With reference standard
<0.5
48
11
1
36
87
36%
(27%, 44%)
12
≥0.5 and <1
44
15
3
26
35
56%
(45%, 67%)
18
≥1 and <2
71
29
1
41
15
83%
(75%, 91%)
30
≥2
299
137
13
149
29
91%
(88%, 94%)
150
Total
462
192
18
252
166
74%
(70%, 77%)
210
*7 patients were excluded from this table due to protocol deviations
**TP: true positive, FP: false positive
***Percent PET positivity = PET positive patients/total patients scanned
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ILLUCCIX is supplied as a kit for preparing Gallium Ga 68 Gozetotide Injection. There are two different kit configurations, each containing 3 vials.
ILLUCCIX Configuration "A" (NDC: 74725-100-25) is intended for use with Ga 68 produced from a cyclotron and purified via GE FASTlab or Eckert & Ziegler GalliaPharm Ge 68/Ga 68 generator and includes:
- Vial 1 (Gozetotide Vial): contains 25 mcg gozetotide and 10 mcg D-mannose as a lyophilized powder in a sterile 10 mL vial with a blue flip-off cap (NDC: 74725-101-25).
- Vial 2 (Acetate Buffer Vial, Configuration A): contains 150 mg anhydrous sodium acetate in 0.292 M HCl solution (2.5 mL volume) in a sterile 10 mL vial with a red flip off cap (NDC: 74725-102-25).
- Vial 3 (Sterile Vacuumed Reaction Vial): an evacuated sterile vial with white flip off cap used to collect Ga 68 chloride from generators or cyclotron and radiolabeling reaction; a multiple-dose vial after radiolabeling.
ILLUCCIX Configuration "B" (NDC: 74725-100-64) is intended for use with Ga 68 produced from an IRE Galli Eo Ge 68/Ga 68 generator and includes:
- Vial 1 (Gozetotide Vial): contains 25 mcg gozetotide and 10 mcg D-mannose as a lyophilized powder in a sterile 10 mL vial with a blue flip-off cap (NDC: 74725-101-25).
- Vial 2 (Acetate Buffer Vial, Configuration B): contains 150 mg anhydrous sodium acetate in 0.175 M HCl solution (6.4 mL volume) in a sterile 10 mL vial with a green flip off cap (NDC: 74725-103-64).
- Vial 3 (Sterile Vacuumed Reaction Vial): an evacuated sterile vial with white flip off cap used to collect Ga 68 chloride from generator and radiolabeling reaction; a multiple-dose vial after radiolabeling.
The radionuclide is not part of the kit. Before radiolabeling with Ga 68, the contents of this kit are not radioactive.
Storage and Handling
Store ILLUCCIX refrigerated upright in the original packaging at 2° to 8°C (36° to 46°F). Do not freeze.
After radiolabeling, keep Gallium Ga 68 Gozetotide Injection upright with appropriate shielding to protect from radiation at ambient temperature (25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)). Use Gallium Ga 68 Gozetotide Injection within 4 hours of preparation.
This preparation is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
-
17 PATIENT COUNSELING INFORMATION
Adequate Hydration
Instruct patients to drink a sufficient amount of water to ensure adequate hydration before their PET study and urge them to drink and urinate as often as possible during the first hours following the administration of Gallium Ga 68 Gozetotide Injection, in order to reduce radiation exposure [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
Manufactured by:
Grand River Aseptic Manufacturing
140 Front Ave SW
Grand Rapids, MI 49504
Distributed by:
Telix Pharmaceuticals (US) Inc.
Fishers, IN 46037
-
PRINCIPAL DISPLAY PANEL - Kit Carton - Configuration A
NDC: 74725-100-25
Rx-Onlyilluccix ®
(kit for the preparation of gallium
Ga 68 gozetotide Injection)25 mcg/vial gozetotide
For Intravenous Use Only
Multiple-Dose KitKit for the preparation of gallium Ga 68 gozetotide Injection
is supplied as multiple dose kit containing:- Prescribing Information
- Diagnostic label
- Syringe label
- Vial 1 (Gozetotide vial) contains 25 mcg gozetotide vial
- Vial 2A (Acetate Buffer Vial) contains acetate buffer
- Vial 3 (Sterile Vacuumed Reaction Vial)
CONFIGURATION:
AConfiguration A is for use with
Ga 68 produced via cyclotron
or EZAG GalliaPharm ®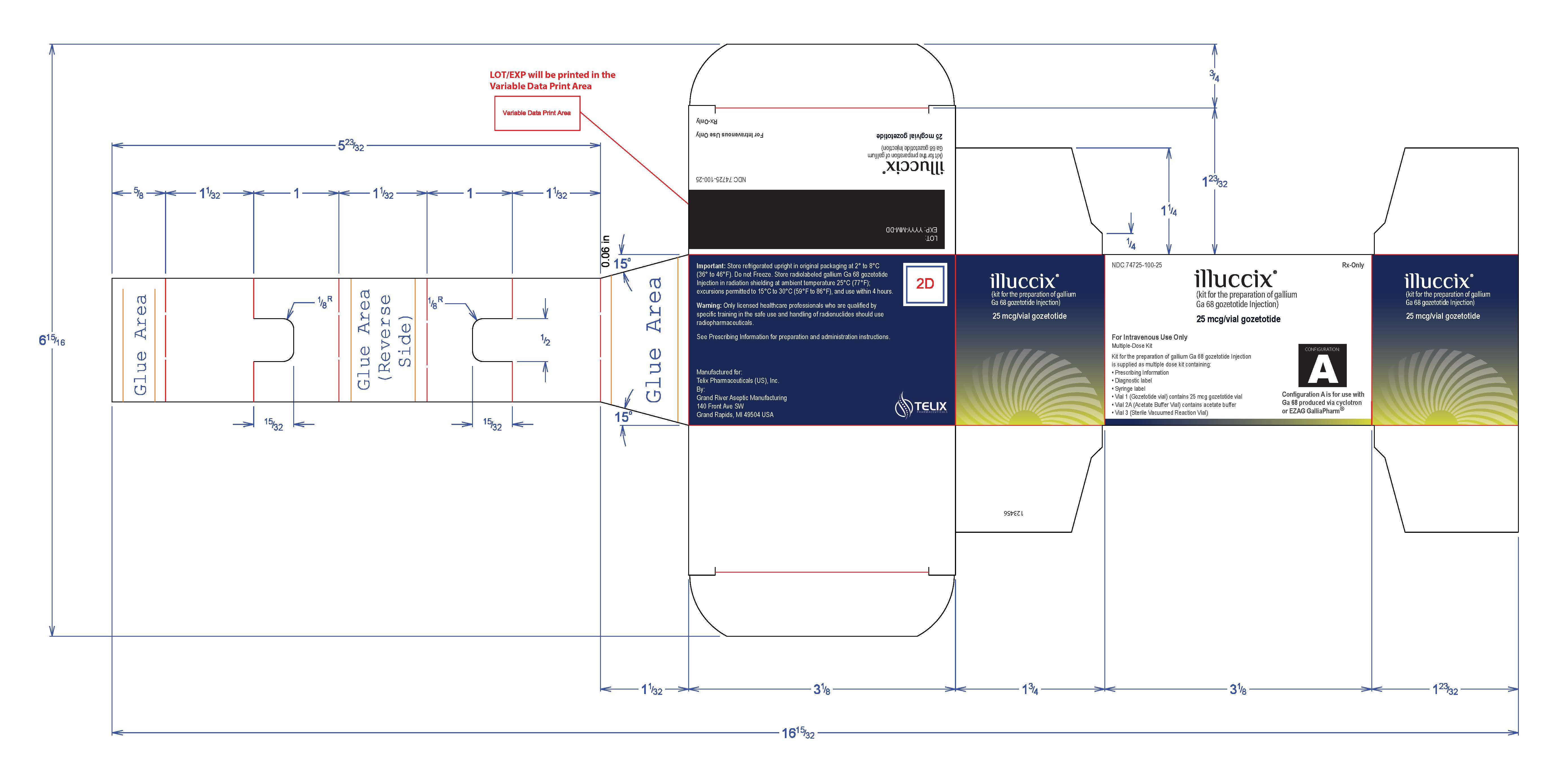
-
PRINCIPAL DISPLAY PANEL - Kit Carton - Configuration B
NDC: 74725-100-64
Rx Only
illuccix ®
(kit for the preparation of gallium
Ga 68 gozetotide Injection)25 mcg/vial gozetotide
For Intravenous Use Only
Multiple-Dose KitKit for the preparation of gallium Ga 68 gozetotide Injection
is supplied as multiple dose kit containing:- Prescribing Information
- Diagnostic label
- Syringe label
- Vial 1 (Gozetotide vial) contains 25 mcg gozetotide vial
- Vial 2B (Acetate Buffer Vial) contains acetate buffer
- Vial 3 (Sterile Vacuumed Reaction Vial)
CONFIGURATION:
BConfiguration B is for
use with IRE Galli Eo ®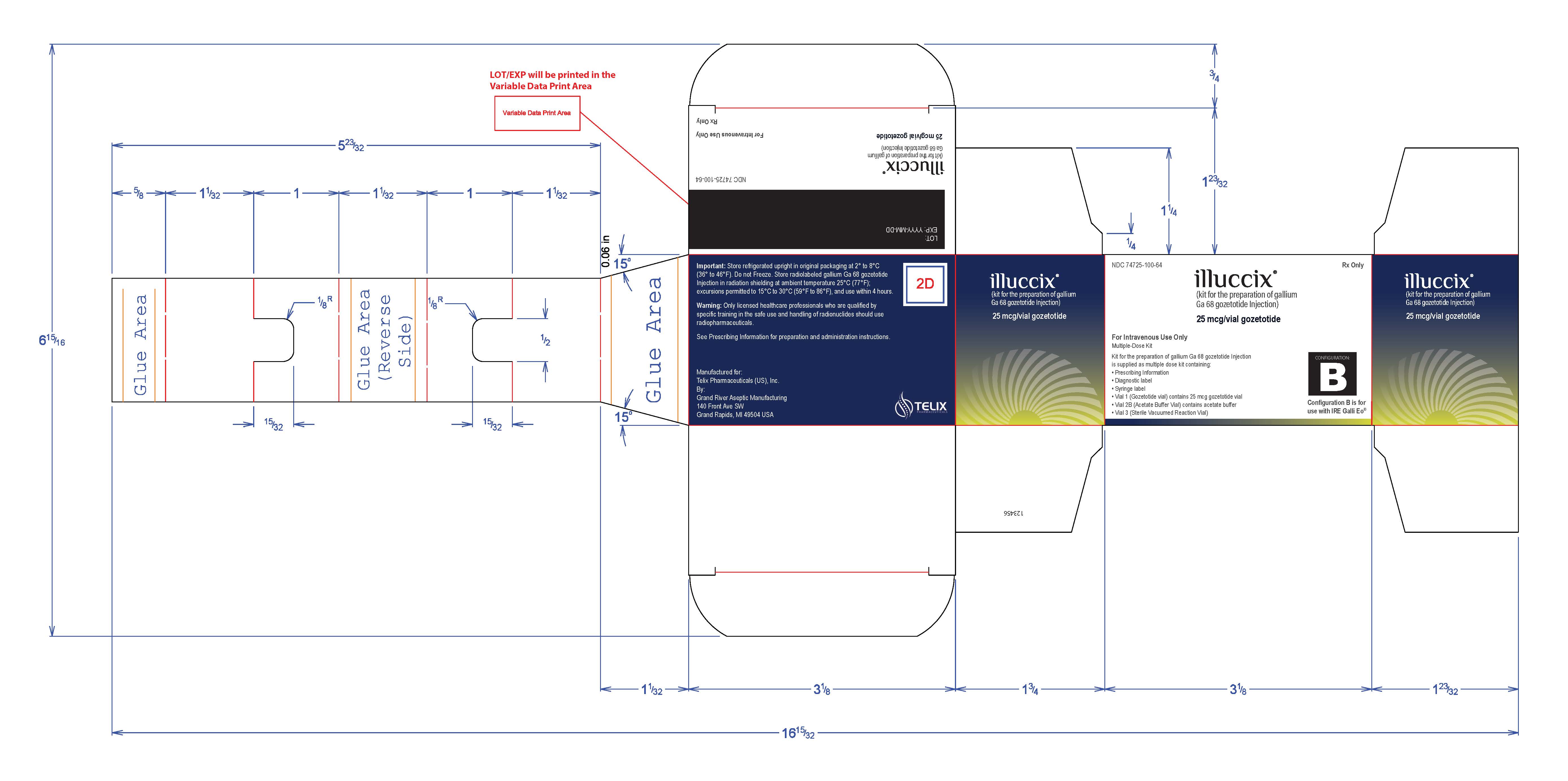
-
INGREDIENTS AND APPEARANCE
ILLUCCIX
kit for the preparation of gallium ga 68 gozetotide injection kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 74725-100 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 74725-100-25 1 in 1 CARTON 12/17/2021 2 NDC: 74725-100-64 1 in 1 CARTON 12/17/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, MULTI-DOSE 1 Part 2 1 VIAL, MULTI-DOSE 2.5 mL Part 3 1 VIAL, MULTI-DOSE 6.4 mL Part 1 of 3 GOZETOTIDE
psma-11 injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 74725-101 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOZETOTIDE (UNII: 9AG41L3AOQ) (GOZETOTIDE - UNII:9AG41L3AOQ) GOZETOTIDE 25 ug Inactive Ingredients Ingredient Name Strength DEMANNOSE (UNII: PHA4727WTP) 10 ug Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 74725-101-25 1 in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214032 12/17/2021 Part 2 of 3 ACETATE BUFFER- CONFIGURATION A
sodium acetate anhydrous solutionProduct Information Item Code (Source) NDC: 74725-102 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM ACETATE ANHYDROUS (UNII: NVG71ZZ7P0) 150 mg in 2.5 mL HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 74725-102-25 2.5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214032 Part 3 of 3 ACETATE BUFFER- CONFIGURATION B
sodium acetate anhydrous solutionProduct Information Item Code (Source) NDC: 74725-103 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM ACETATE ANHYDROUS (UNII: NVG71ZZ7P0) 150 mg in 6.4 mL HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 74725-103-64 6.4 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214032 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214032 12/17/2021 Labeler - Telix Pharmaceuticals (US) Inc. (116991792) Establishment Name Address ID/FEI Business Operations Telix Innovations 370765512 analysis(74725-101)
Trademark Results [ILLUCCIX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ILLUCCIX 79297402 not registered Live/Pending |
Telix Pharmaceuticals Limited 2020-08-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.