Preparation H Suppositories(cocoa butter and phenylephrine HCl)
Major Prep Hemorrhoidal by
Drug Labeling and Warnings
Major Prep Hemorrhoidal by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
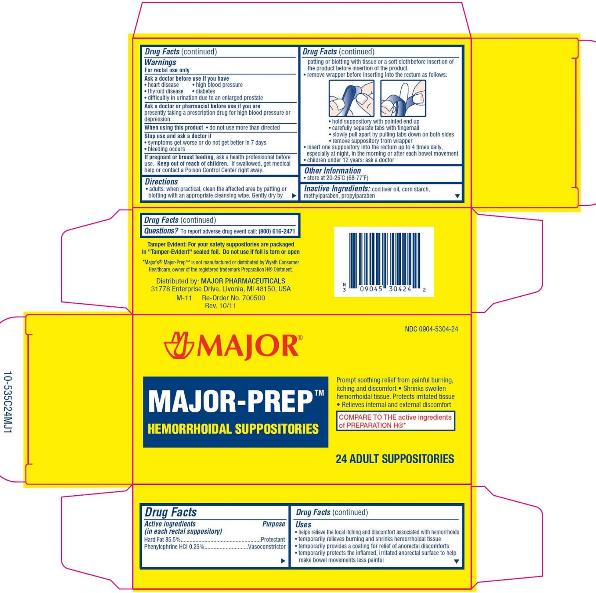
MAJOR PREP HEMORRHOIDAL- hard fat, phenylephrine hcl suppository
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Preparation H Suppositories
(cocoa butter and phenylephrine HCl)
USES
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporarily relieves burning and shrinks hemorrhoidal tissue
- temporarily provides a coating for relief of anorectal discomforts
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
WARNINGS
For rectal use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
DIRECTIONS
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before insertion of this product.
-
detach one suppository from the strip; remove the foil wrapper before inserting into the rectum as follows:
- hold suppository with rounded end up
- as shown, carefully separate foil tabs by inserting tip of fingernail at end marked “peel down”
- slowly and evenly peel apart (do not tear) foil by pulling tabs down both sides, to expose the suppository
- remove exposed suppository from wrapper
- insert one suppository into the rectum up to 4 times daily, especially at night, in the morning or after each bowel movement
- children under 12 years of age: ask a doctor
PRODUCT PACKAGING
MAJOR-PREP HEMORRHOIDAL SUPPOSITORIES
Reduces Internal Swelling. Soothes and Protects
- Prompt Soothing Relief from Painful Burning, Itching and Discomfort
- Shrinks Swollen Hemorrhoidal Tissue
- Protects Irritated Tissue
- Also for Nighttime Relief
24 SUPPOSITORIES
Individually sealed for your protection.
Do Not Use if foil is torn or open.
Distributed by
Major Pharmaceuticals
Livonia, MI 48150 USA
| MAJOR PREP HEMORRHOIDAL
hard fat, phenylephrine hcl suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
Revised: 1/2020
Document Id: ed4e822e-03a4-40e8-baf7-91e75a873320
Set id: d47f9fa8-af7b-46c5-af27-3fa1eb5e26da
Version: 4
Effective Time: 20200102
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.